Surface Finishes
Aluminum Anodizing Services
We offer anodizing type Ⅱ and type Ⅲ, which are ideal surface finishes for aluminum parts. Anodizing protects aluminum parts from wear and corrosion.
Aluminum Anodizing
Anodizing is an electrolytic passivation procedure that grows the natural oxide layer on aluminum components for security from wear and corrosion, along with aesthetic results. It is a conversion covering, similar to Alodine, meaning that the surface area of the lightweight aluminum recedes dimensionally before the safety oxide layer is developed. After the procedure is full, the oxide layer is integral to the lightweight aluminum substrate below, which means it will not chip or flake.
| Anodize Type | Applicable Materials | Surface Preparation | Color | Glossiness | Cosmetic Availability | Thickness | Visual Appearance |
| Type Il Anodize | Aluminum | As machined (Ra 1.6μm) | Clear, Black, Red, Blue, Purple, Gold | Matte | No | 50μm to 150μm | Parts are anodized directly after machining. Machining marks will be visible. |
| Bead blasted (Grit #120 #150 #200) | Clear, Black, Red, Blue, Purple, Gold | Matte | On Request | 8μm to 16μm | Grainy texture, matte finish | ||
| Type Ⅲ Anodize | As machined (Ra 1.6μm) | Clear,Black | Matte | No | 35μm to 50μm | Parts are anodized directly after machining. Machining marks will be visible. | |
| Bead blasted (Grit #120 #150 #200) | Clear,Black | Matte | On Request | 35μm to 50μm | Can be slightly visible if parts are “Not Cosmetic” Completely removed if parts are “Cosmetic” |
Aluminum Anodizing Type II Color Options

Clear/Natural

Black

Gold

Green

Purple

Red

Blue

Custom
Aluminum Anodizing Type Ⅲ Color Options

Natural

Black
- *Anodizing is often used with the beadblasting to give the surface a uniform appearance.
- *Glossy anodizing is available on request.
- * If you need a particular Pantone color code, please contact info@ecoreprap.com.
Aluminum Anodizing Parts

Anodizing Type II Red

Anodize Type II Red Detail

Anodizing Type Ⅲ Natural

Anodizing Type Ⅲ Natural Detail
Anodizing Process
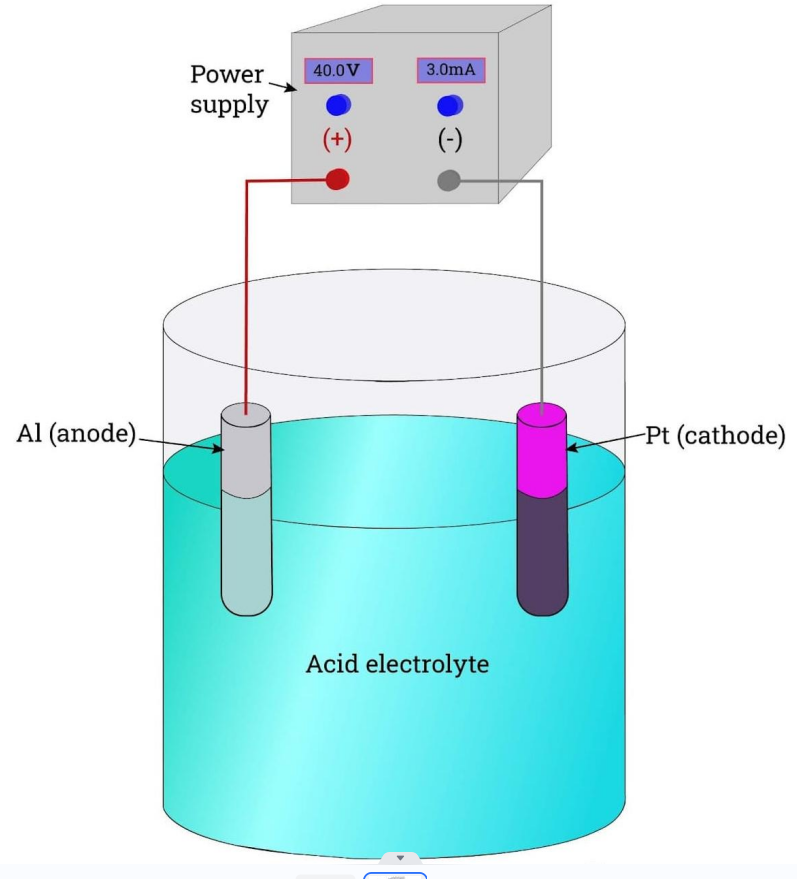
Anodizing is an electrolytic passivation treatment that grows the all-natural oxide layer on aluminum parts for security from wear and rust, along with aesthetic outcomes. Let us break down the basic steps for anodizing a part:
1. Connect the part to an anode
2. Submerge the part in acidic electrolyte solution
3. Apply a cathode to a metal electrode in the solution
4. Positive ions react with negative O2 ions and the surface becomes porous
5. This grows a layer of aluminum oxide on the part
6. Use corrosion inhibitors or colored dyes for cosmetic purposes
7. Seal the nanopores
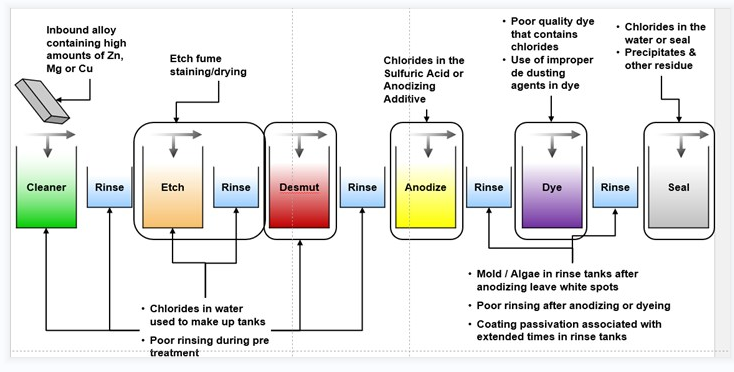
Aluminum Anodizing Design Considerations
- Anodizing uses jigs or racks to hold parts, which can leave marks in untreated areas. If you need specific areas mark-free, provide a drawing and follow our cosmetic procedure.
- Anodizing insulates your parts, reducing their conductivity.
- Anodizing doesn't require masking by default because it doesn't significantly increase part thickness. If you need specific areas masked or plugged, please specify that in your CAD file.
- Type III anodizing significantly thickens your part's surface. To maintain critical surfaces, we advise plugging or masking threaded/reamed holes and other vital areas.
FAQs
Anodizing is an electrochemical process that forms a protective oxide layer on the surface of metal, typically aluminum, to enhance corrosion resistance and improve surface durability.
No, anodizing is primarily used for aluminum and its alloys. It is not suitable for most other metals like steel or copper.
The two primary types are sulfuric acid anodizing (Type II) and hard anodizing (Type III), with Type II providing a thinner and decorative finish and Type III producing a thicker, more wear-resistant coating.
Anodizing provides corrosion resistance, enhances aesthetics, and can be used to prepare the surface for coloring, making it a common choice for aluminum products.
Yes, anodized aluminum parts can be dyed to achieve various colors. The porous nature of the anodic layer allows for dye absorption.
The two primary types are sulfuric acid anodizing (Type II) and hard anodizing (Type III), with Type II providing a thinner and decorative finish and Type III producing a thicker, more wear-resistant coating.
Yes, anodizing can reduce the electrical conductivity of aluminum. The anodizing process creates an insulating oxide layer on the surface of the aluminum, which can hinder its electrical conductivity. The extent of the reduction in conductivity depends on the thickness of the anodic layer and the specific type of anodizing process used.
Yes, anodizing is suitable for both intricate and precision parts, as it offers good dimensional control. For highly precise dimensions, especially with Type III anodizing, it’s important to note that it may cause size changes.