- Capabilities
-

CNC Machining
Tight tolerance and 20+ finishes 3, 4 , 5 axis, as fast as 2 days -
Sheet Metal Fabrication
High-precision, on-demand sheet metal cutting and bending. -
3D Printing
SLA, SLS,MJF,SLM, FDM 3d printing with post treatment. -

Vacuum Casting
Production quality parts without the tooling investment.
-
- Solutions
Rapid Prototyping
Fastest lead time of high-quality prototypes at minimal cost.
Low Volume Production
From one-off prototyping to low-volume production.

Mechanical Assembly
Custom assembly for project-specific needs.

Custom Package
Ready to help you prompt your brand.
- Sources

Materials
Select from 100 more types of metals and plastics.
Finishes
Select from 20 more types of surface fishes.
Industries
Providing precision machining and manufacturing solutions.

Cases
How we assist our clients in bringing their projects to fruition.
- Company
Quality Assurance
Consistent quality, every time.

About Us
Your go-to manufacturer for custom parts.

Newsroom
Learn updated news about ECOREPRAP.
Surface Finishes
Electropolishing Services
Electropolishing is an electrochemical process utilized to enhance the surface area coating of a part by removing material to level microscopic optimal and valleys.
All your designs are secure and confidential
Electropolishing
Electropolishing is an electrochemical procedure used to boost the surface coating of a component by removing material to level microscopic optimal and valleys. This process can brighten, passivate, and deburr the parts. This process works the opposite way than that of plating procedures, as the part works as the anode in the reaction. As the present travels through the part (anode), the surface area is oxidized and liquified in the service to the cathode. Electropolishing is extremely helpful in polishing uneven parts with difficult to hard-to-reach surfaces. Additionally, just a percentage of the product is removed, so resistance will certainly not be greatly affected by this procedure.
| Applicable Materials | Cosmetic Availability | Visual Appearance |
| Stainless steel, Aluminum, Copper, Brass | NA | Smooth, glossy finish |
Electropolishing Parts


Electropolishing Process
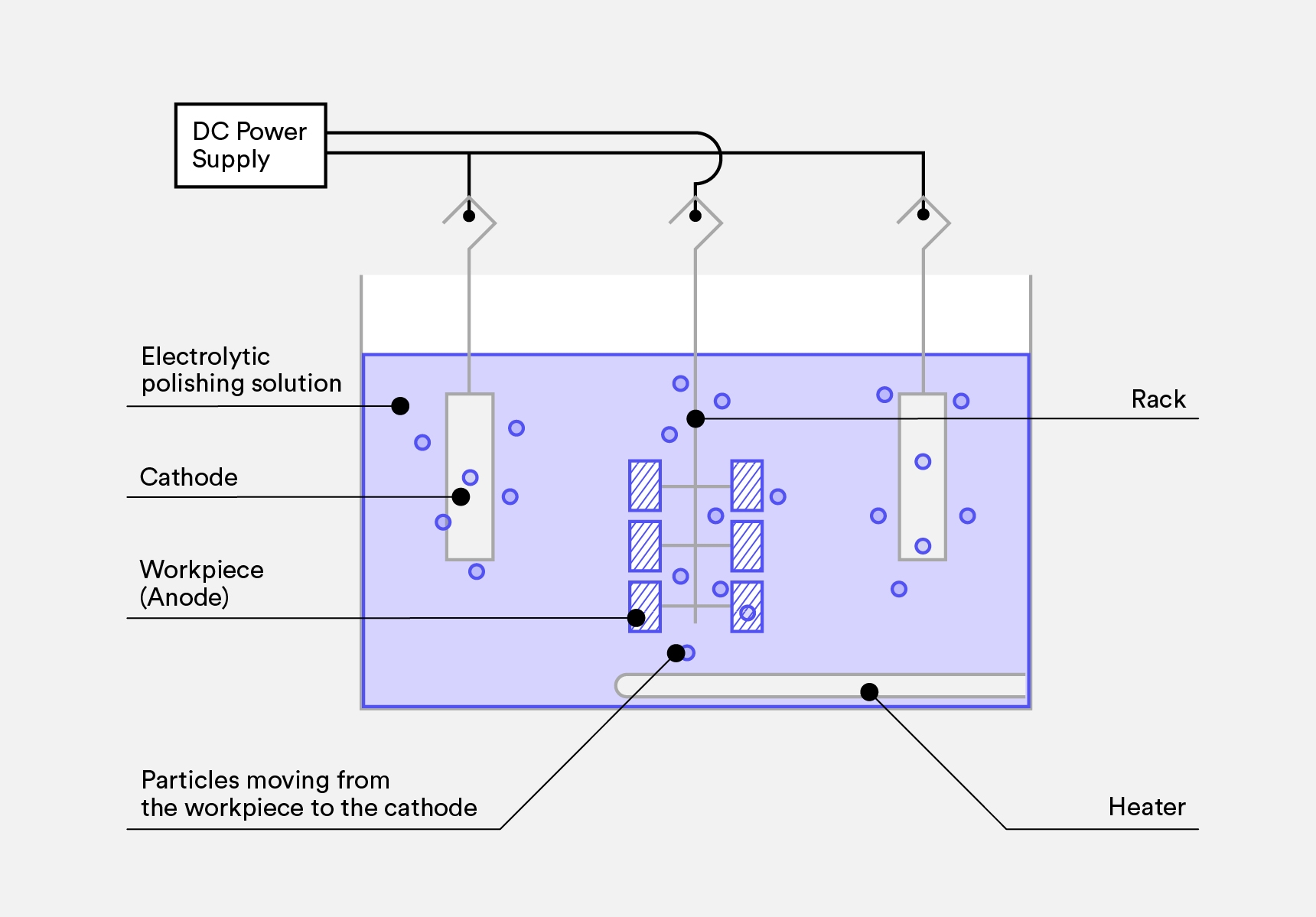
Here’s how to apply brushing and electropolishing:
- The Part’s surface is cleaned and prepared by power brushing.
- The metal part being electropolished (aka anode) is connected to the positive terminal of a direct current (DC) rectifier and submerged in a tank of an electrolyte solution. The unfavorable terminal of the rectifier is connected to a cathode (typically stainless steel) that is likewise submerged in the tank.
- A current runs through the solution from the anode to the cathode, causing ions on the surface of the material to oxidize and dissolve into the solution.
- Parts are then rinsed from the electrolyte solution in a different tank.
- To finalize the finish, parts go through a final post-dip in a Nitric dip or a Citric acid, rinsed with cold water and are put to dry.
Electropolishing Design Considerations
Masking:Electropolishing does not have a significant dimensional impact, therefore masking parts is not necessary.
FAQs
Electropolishing is an electrochemical process that removes a controlled amount of surface material to improve the finish, corrosion resistance, and cleanliness of metal parts.
Electropolishing enhances the appearance, passivates the surface, and improves corrosion resistance, making it an attractive finish for stainless steel and other metals.
Electropolishing is primarily used for stainless steel, but it can also be applied to other metals such as aluminum, copper, and brass.
Electropolishing typically removes a very thin layer of material, resulting in minimal dimensional changes, suitable for precision components.
Yes, electropolishing is effective on parts with complex shapes, fine details, and intricate geometries.
Electropolishing can be combined with other processes, such as passivation or coating, to achieve specific performance and appearance requirements.
Electropolishing improves the electrical conductivity of metal parts and does not provide electrical insulation.
Post-electropolishing treatments like passivation may be required for specific applications to enhance corrosion resistance.
Electropolishing can be environmentally friendly when using eco-friendly electrolytes and efficient wastewater treatment.
Electropolishing produces a smooth, bright, and reflective surface finish with reduced micro-roughness, typically with an Ra value of less than 0.2 micrometers (渭m).