Table of Contents
Chrome plating is a type of popularly used finishing method for various applications. There are many common things in our life that are plated with a chrome coating.
Chrome-coated parts are usually bright, wear-resistant and rust-proof. So, what is chrome plating, and what influence does it have?
This article will introduce everything you should know about chrome plating.
Key Takeaways:
- Chrome plating, or chromium electroplating, is a surface finishing technology that deposits a layer of chromium onto a metal or plastic surface through an electrolytic process.
- Chrome plating provides three main benefits: decorative appeal (a bright, mirror-like finish), enhanced surface hardness, and superior corrosion and wear resistance.
- Chrome plating process is divided into three main procedures: pre-process, electroplating of chrome, and post-process, with pre-process being essential for proper adhesion and coating quality.
- The two main functional types are Decorative Chrome and Hard Chrome or Engineering Chrome, with all plating baths requiring materials like Chromic Acid (CrO3) and Sulfuric Acid (H2SO4) to work.
- A significant consideration is that hexavalent chromium plating, a traditional method, uses Cr⁶⁺, a potent carcinogen posing serious health and environmental risks.
1. What is Chrome Plating?
Chrome plating, also known as chromium plating or chrome electroplating, is a surface finishing technology that deposits a layer of chromium onto a metal or plastic surface through an electrolytic process.
The diagram below illustrates the principle of electroplating. For chrome plating, the metal ions are chromium.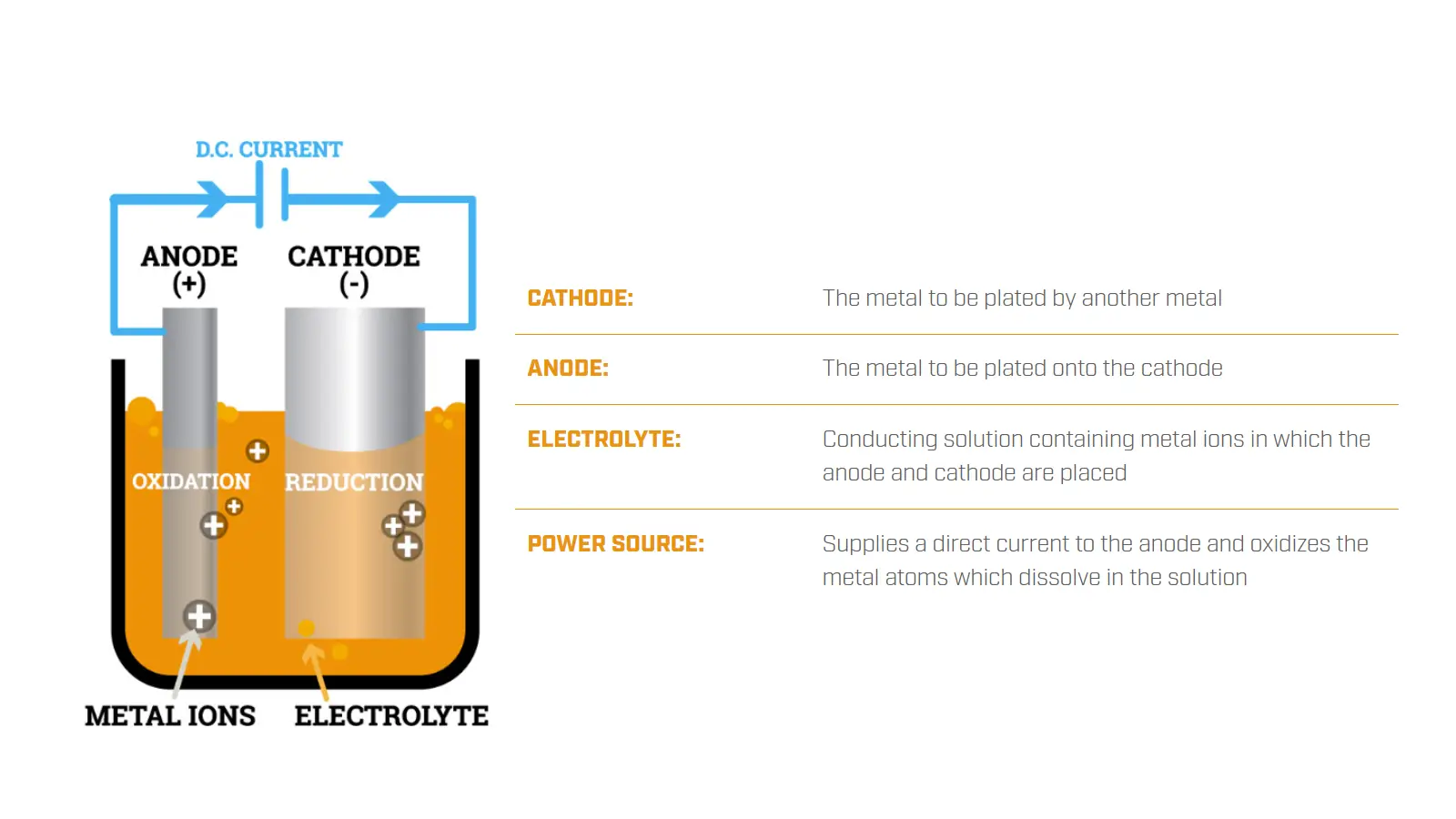
Chromium itself is a silver-white metal characterized by its inherent good hardness and corrosion resistance.
Chrome coating can provide the plated parts with decorative appeal, enhanced surface hardness, and excellent corrosion resistance.
Chrome plating is widely used across industries, including CNC machining, specifically to improve the quality and performance of machined components.
Chromium is discovered to be able to serve as a protective coating in the early 20th century. And in the 1920s scientists developed chrome electroplating technique. Then chrome plating came into commerce for its appealing appearance and excellent resistance to rust and abrasion.
With continuous development, chrome plating made progress with emergence of different types such as hard chrome and decorative chrome, meeting specific industry requirements.
Even today, chrome plating remains one of the most popular finishing methods in manufacturing, successfully making components more durable and visually attractive.
2. How Does Chrome Plating Work?
It is essential to monitor and control key parameters such as thickness, temperature, current density, and Chromic Acid concentration during the plating process to achieve consistent quality and desired performance.
Below photos shows some parts under chrome plating in a workshop.
2.1. What Chemical Materials Does Chrome Plating Need?
Chromic Acid (Chromium Trioxide – CrO₃): It is the primary component of plating baths for chromium coatings. It provides the chromium ions for the
Sulfuric Acid (H₂SO₄): Mixed with chromic acid to balance the plating bath chemistry, it helps to control the thickness and uniformity of the entire coating.
Hydrochloric Acid (HCl): It is used during the activation stage to remove oxides and other surface contaminants from the surface and prepare it for plating, which would promote the adhesion of the chromium layer.
Sodium Hydroxide (NaOH): This chemical is commonly used to clean grease, oil, and other organic contaminants off from the part before it is placed into the plating bath.
Fume Suppressants: They are usually added to the plating bath for reducing release of toxic mists, especially in hexavalent chromium plating, which is important for safety and environmental friendliness.
Additives(Hydrators and Catalysts): Chemicals such as sulfate concentrates and wetting agents can help to achieve the desired appearance as well as hardness, and to reduce defects.
Buffers: They are used to stabilize the pH of the plating solutions and to ensure the electroplating conditions under certain control. Proper buffering is essential for consistent coating quality.
Water (H₂O): Water is used to dilute the plating baths and keeps its concentration within correct level. It also plays a critical role in rinsing process during different stages.
2.2. What Equipment Does Chrome Plating Need?
Degreasing Tanks: For cleaning oils, dirt, and other impurities from parts before plating.
Rinse Tanks: For washing off residual chemicals between different stages of the plating process.
Acid Bath Tanks: For activating and micro-etching the surface in preparation for plating, typically containing sulfuric acid or hydrochloric acid.
Chromium Plating Tanks: Exactly for chromium plating, containing chromic acid solution and fume suppressants to control hazardous emissions.
Rectifiers: For supplying and regulating the current required for the electroplating process, with the flow of electricity to the plating tank controlled.
Anodes (Lead or Graphite): For attracting the chrome particles in the bath to the plating part and ensuring uniform chromium deposition.
Filtration System: For keeping the plating solution free from contamination. Regular filtration can prevent impurities from degrading plating quality.
Ventilation System: For management of hazardous fumes generated during the plating, critical for safety and environmental compliance.
Polishing and Grinding Equipment: For plating preparation or finishing to make the surface smooth and
Temperature Control System: For maintaining the plating bath at the required temperature. Consistent temperature is crucial for achieving uniform chromium coatings.
Personal Protective Equipment (PPE): For the safety of operators, including professional gloves, masks, and protective suits to handle chemicals.
Waste Treatment System: For treating and neutralizing chemical waste produced during the plating process. It is critical for safe disposal and proper environmental compliance.
2.3. What is the Process of Chrome Plating?
The process of chrome plating can be divided into three main procedures, including pre-process, electroplating of chrome, and post-process. And these three procedures cover certain detailed steps.
Pre-process: The purpose of pre-process is to make the surface of parts completely clean and properly smooth, thus to make the coating stably plated on the surface. It is the essentially primary procedure since any oil, oxide, or impurity would cause blistering, peeling, or spotting in the final coating.
- Polishing/Grinding: Mechanical polishing or grinding is performed first to eliminate surface imperfections such as scratches and burrs, to make thesurface smooth and flat. This step is for aesthetics and uniform electrodeposition in subsequent plating stages.
- Sandblasting: It is used for parts requirea specific matte finish or enhanced adhesion.
- Degreasing: During this step, the part would beimmersed in a hot alkaline degreasing solution, where saponification and emulsification reactions would thoroughly remove oils, lubricants, and residual polishing compounds.
- Rinsing: After every chemical treatment, the part must be thoroughly rinsed with clean water (typically flowing water) to prevent cross-contamination between baths, whichis crucial for plating quality.
- Acid Pickling: Firstly, immerse the part in a strong acid to remove rust, scale, and heavy oxide layers. And then it is required to dip the part briefly in a mild acid solution immediately before It is also essential for excellent adhesion.
- Pre-plating: For metal parts, copper coating and nickel coating are usually plated on the surface before chrome plating. Copper coating is used to cover the defects of the surface while nickel coating serve as the primary corrosion barrier.
For plastic, a thin conductive metal layer(usually copper or nickel) should be plated by electroless process first.
Chrome Electroplating: After pre-process, the part is ready to be electroplated.
- Racking: The part should besecurely mounted onto a specialized plating rack to ensure uniform electrical contact across all surfaces. The design of the rack is critical to achieving consistent plating thickness and quality.
- Electroplating: Immerse the rack, withthe part attached, into the chromium electroplating And then turn on the current, with the rack connected to the negative terminal as the cathode and the anode, typically made of insoluble lead or lead alloy connected to the positive terminal.
Under the influence of direct current (DC), chromate ions in the electrolyte would be reduced at the cathode and deposit to be a chrome coating.
Post-process: It is usually for cleaning and special treatment.
- Recovery Rinsing: Remove the part form the plating tank and put it into the recovery rinse tank to reclaimthe expensive chromium baths adhering to the surface.
- Rinsing: Rinse the part with pure water to remove all residual plating chemicals.
- Drying: Immediately dry the part by hot air to prevent water spots or rust marks on the freshly plated surface.
- Inspecting: checking the appearance, thickness, adhesion, and corrosion resistance etc.
2.4. What are the Critical Parameters during Chrome Plating?
For great quality and desire performance, it is essential to carefully monitor and control multiple key parameters during the chrome plating process.
These factors directly determine the corrosion resistance, hardness, appearance, and abrasion resistance etc. The critical parameters during chrome plating are as follows.
- Thickness: The thickness of the chrome coating determines its durability and functionality. Hard chrome plating typically requires a thicker coating to enhance wear resistance, while decorative chrome plating requires a thinner layer to improve appearance.
- Temperature: Maintaining a stable temperature is crucial since temperature affects the plating rate, coating uniformity, and overall quality. It is required to precisely control the bath temperature for consistent results, particularly during hard chrome
- Current Density: The current applied during the process influences how chromium deposits onto the surface. Correct current density is essential for uniform thickness and strong adhesion.
- Chromic Acid Concentration: The concentration of chromic acid in the plating solution is critical to the plating quality. Improper concentration can lead to defects such as pitting, roughness, or poor adhesion.
- Sulfate Concentration :It is vital to balance the sulfate concentration with the chromic acid, which helps to control the bath chemistry, ensure a stable plating process, and prevent issues like micro-cracking.
- Bath Agitation: Proper agitation during plating can prevent contaminant deposition on the part and ensure a uniform coating. Mechanical stirring or air bubbles are two common ways for it.
- Anode Material and Placement: The deposition rate and quality would be essentially influenced by the type and placement of anodes. The most common types are lead or graphite anodes, depending on the specific plating requirements.
- Surface Preparation: The whole pre-process is vital to make the part free of contaminants, which is necessary for effective adhesion and surface smoothness.
- Plating Time: The plating time affects the thickness of the chromium coating. A longer time means a thicker coating, resulting in better durability and wear resistance.
3. What are the Main Types of Chrome Plating?
There are various types of chrome plating in manufacturing. Each of them would provide specific functions. It is critical to know them clearly and use them properly. This session will introduce the main types of chrome plating from the function, plating solution, and coating structure.
3.1. Classification of Chrome Plating By Function
Decorative Chrome Plating
Decorative chrome plating is primarily applied to enhance product aesthetics, achieving an attractive look through its bright, shiny, and reflective finish.
Decorative chrome plating can provide basic corrosion resistance and abrasion resistance. But its primary function is decoration.

Features of Decorative Chrome Plating
- High Reflectivity: Decorative chromium plating offers one of the highest reflectivity levels among all electroplated coatings, producing a bright, clear mirror-like reflection with a visually luxurious appearance.
- Bluish-White Luster: Unlike the slightly yellowish tones of stainless steel or nickel, chromium exhibits a unique, cool bluish-white luster, showing modern and high-end.
- Excellent Chemical Stability: It remains highly stable in air, resisting discoloration, tarnishing, and corrosion from sweat, humid air, and certain chemicals.
- Multiple Layers: Decorative chrome plating is not a single chromiumlayer, but a composite structure consisting of a base copper layer, a middle nickel layer, and a top chromium layer.
- Extremely Thin Coating: Since it is for decoration but not functions, decorative chromium plating is typically very thin, generally ranging from 0.25 to 1 micron.And this can reduce cost.
Process of Decorative Chrome Plating
- Pre-process: This is the most critical step, includingpolishing, degreasing, rust cleaning and activation, to ensure the surface is absolutely clean and smooth, since any imperfections will be magnified after plating.
- Plating the Base Layer(Copper Layer): With excellent ductility, copper can effectively cover minor defects on the surface and provide a significantly smooth and level foundation.
- Plating the Middle Layer(Nickel Layer): It is nickel coating that actually provides corrosion resistance of decorative chrome plating. Meanwhile, the nickel coating is the thickest layer within the decorative chrome plating. Since the chromium layer is micro-porous, directly plating it onto the part would lead to rapid corrosion. Serving as a barrier, the dense nickel layer can prevent the part from rust.
- Plating the Top Layer(Chrome Layer): Place the pre-processed part into a chromium plating solution containing chromic anhydride, sulfuric acid, and catalysts for electroplating.By plating a very thin chromium layer onto the smooth nickel surface, the final finish would show with mirror-like luster and bluish-white hue.
- Post-process: Passivate and seal the final coating to enhance the corrosion resistance and colorfastness. And the subsequent processes include film removal, polishing, drying, visual inspection, thickness measurement, and performance testing.
Advantages of Decorative Chrome Plating
- Appealing Appearance: Decorative chrome plating can impart a mirror-like luster to theplated parts and enhance their visual appeal and texture, thereby making them more attractive.
- Good Durability: Though the coating is very thin, decorative chrome plating is still corrosion-resistant and wear-resistant. In addition, its attractive appearance can remain long time.
- Easy to Clean: The smooth surface repels dirt and is easy to remain bright simply by wiping.
- Disadvantages of Decorative Chrome Plating
- Higher Cost: The requirement of multiplecoatings makes the process more complex and consuming, leading to higher cost.
- Tight Require for Pre-process: The pre-process of decorative chrome plating should be nearly flawless, otherwise issues suchas blistering and peeling would occur.
- Irreparability:Once the decorative chrome plating is scratched by a hard object and then the middle nickel coating is exposed, corrosion will start from the scratch and spread to the surrounding area. What’s more, this cannot be easily repaired.
Hard Chrome Plating
Hard Chrome Plating (or Engineering Chrome) refers to a thicker chromium coating applied primarily to enhance the surface performance of metal components due to its superior hardness (HV800–1200) and wear resistance.
Compared with decorative chrome plating commonly seen in daily life, hard chrome plating is primarily to enhance the surface performance of metal components, rather than merely for aesthetic appeal.
Features of Hard Chrome Plating
- Superior Hardness: The coating hardness can reach HV800–1200, close to that of quenched tool steel.
- Excellent Wear Resistance: Hard chrome plating performs exceptionally well under both dry and lubricated friction conditions, making it suitable for environments within high level abrasion such as hydraulic cylinders, piston rods, and molds.
- Low Friction Coefficient: The surface is smooth and dense, making itself withlow friction coefficient and good self-lubricating propert Therefore, it can help moving parts to reduce abrasion.
- Good Corrosion Resistance: Although not as corrosion-resistant as stainless steel, hard chrome plating still offers decent resistance in atmospheric environments, fresh water, and oil media.
- Reparability: Since the thickness of hard chromium coating can be precisely controlled, the coating can be used to repair parts that have become dimensionally out of specification due to wear or machining errors. The coating can restorethem to their original dimensions at a cost significantly lower than replacement.
- Good Resistance for Heat: Hard chromiumplating maintains stable performance at temperatures up to 500°C, with short-term tolerance for even higher temperatures.
- Strong Adhesion: With proper processes, the hard chromium layer would bond firmly withthe base metal, making it resistant to peeling.
- Process of Hard Chrome Plating
- Pre-process: This is the most critical step, directly determining the quality of the coating. Firstly, polish and grind the surface to ensure it smooth. Secondly, thoroughly removeoil and other contaminants from the surface. Thirdly, rinse the part with clean water. Finally, activate the part by acid pickling to remove the thin oxide layer on the surface, thus ensuring strong adhesion between the coating and the part.
- Electroplating: Immerse the pre-processed part, as the cathode, into an electroplating solution containing chromic anhydride and catalysts. Then apply direct current to the solution and part. The hexavalent chromium ions in the solution wouldgain electrons at the cathode and reduce to metallic chromium, and finally deposit onto the part.
- Post-process: Remove residual plating solution from the In addition, for critical load-bearing components, heating(180–200°C) for several hours is required within a specific period after plating to remove hydrogen.Finally, grind or polish the surface as required to achieve higher dimensional accuracy and surface smoothness.
Classification of Hard Chrome Plating
Porous Chrome Plating
Porous chrome plating is essentially ceased by spot corrosion. The process has two steps. The first step is to adjust the concentration of catalyst and current density to plate a chromium layer with high internal stress and numerous micro-cracks on the surface.
And the second step is to immerse the plated part in the chromium plating solution or a dedicated electrolyte as the anode and apply an electric current. Due to the micro-cracks in the chromium layer, chromium at the crack edges would preferentially dissolve during anodic dissolution, thereby widening and deepening the micro-cracks to form a network of grooves.
- Surface Morphology: Macroscopically, the surface appears dull grayish-white and lacks luster.While under a microscope, the surface consists of a network or crazing-like structure composed of countless grooves and plateaus. The grooves serve as oil reservoirs, and the plateaus are the primary load-bearing areas.
Key Features:
- High Oil Retention: The groove structure provides exceptional oil-holding capacity.
- Excellent Running-in Performance: During initial operation, the higher plateaus are slightly worn down first, which would increase the effective load-bearing area and improve mating performance.
- Outstanding Anti-Galling Property: Particularly suitable for heavy-load, slow speed, heat, and reciprocating motion applications where lubrication conditions may be poor.
Typical Applications: Engine cylinder liners(especiallyfor large diesel engines), piston rings, journal surfaces of large crankshafts and other sliding friction components in heavy machinery.
Microporous Chrome Plating
- Process:
- Composite Coating Method: Before chromium plating, first deposit a nickel or nickel alloy underlayer withnon-conductive fine particles such as alumina, silica, diamond powder, and then apply a hard chromium coating. Subsequently, grind or polish the surface to expose the embedded particles, and remove them by acid etching to form micropores.
- Laser/Mechanical Drilling Method: After electroplating the hard chromium coating, drillmicropores on the surface by laser or mechanical tools.
- Electrochemical Etching Method: Generate a microporous structure on the coating surface through selective electrochemical corrosion.
- Surface Morphology: Macroscopically, the surface is smoother than porous chromium plating.While under a microscope, the surface exhibits uniformly distributed dot-like pits with extremely high density, but not continuous network-like grooves.
Key Features:
- High Densityof Pore: The number of pores far exceeds that of porous chromium plating. And they are distributed far uniformly.
- Uniform Oil Film: The dot-like micropores enable the lubricating oil film to spread evenly across the entire friction surface, resulting in more stable lubrication performance.
- Minimal Initial Wear: Due to larger and flatter surface plateaus without sharp edges, the wear during initial operation is significantly reduced.
- Superior Wear Resistance: Especially suitable for high-speed, light-to-medium load applications with requirements of high precision and long service life.
Typical Applications: Piston rings in high-performance engines, plungers and cylinder barrels in hydraulic systems, guideways in precision machine toolsand other precise components tight to tribological performance.
Micro-cracked Chrome Plating
Process:
Method 1. Use chromium plating solutions containing fluorides or organic additives to promote increased internal stress and induce crack formation.
Method 2. Turn the current density high and temperature low to elevate internal stress within the coating, thus encouraging natural cracking.
Method 3. Induce cracks after plating through heat treatment or mechanical stress.
- Surface Morphology: Macroscopically, it is virtually similar to decorative chromeplating, exhibiting the same bright mirror-like appearance that cannot be differentiated by the naked eye.
While under a microscope, the surface reveals an extremely fine, continuous, and irregular network of micro-cracks. And the crack widths are extremely narrow, typically less than 0.5 micrometers.
Key Features:
The key advantages of chrome plating include exceptional corrosion resistance, high surface hardness for improved durability, and a visually appealing, easy-to-clean finish.
- Excellent Corrosion Resistance: This is its primary advantage. By dispersing corrosion current, the corrosion resistance of micro-cracked chromium plating under harsh conditionsis several times than that of decorative chromium coating.
- Decorative Appearance: Micro-cracked chrome plating fully retains the bright and lustrous appeal meanwhile enhancing performance of the surface.
- Improved Lubricity: The dense micro-crack network provides certainoil retention, resulting in a lower coefficient of friction and improved wear resistance compared to conventional chromium.
Typical Applications: Micro-cracked chromium plating is primarily used in applications demanding both premium aesthetics and exceptional corrosion resistance, especially for components exposed outdoor. It is mostly applied to automotive exterior trim. It is also usually used on bathroom fixtures, outdoor hardware, piston rods in aircraft engines, rollers in high-speed printing presses, and guideways in precision machine tools.
Table of Comparison: Porous VS Micro-porous VS Micro-cracked Chrome Plating
| Feature | Porous Chrome Plating | Micro-porous Chrome Plating | Micro-cracked Chrome Plating |
|---|---|---|---|
| Pore/Crack Size | tens to hundreds μm | 10–30 μm, uniformly distributed | Crack width<1μm, depth<10μm, network pattern |
| Density | Sparse | High(10⁴-10⁵pores/cm²) | High(400–1000 cracks/cm) |
| Roughness | High(Ra > 1 μm) | Medium(Ra 0.2–0.8μm) | Low(Ra 0.1–0.4 μm) |
| Oil Retention | ★★★★☆(Large pores hold more oil) | ★★★★☆(Uniform pores, even oil retention) | ★★★☆☆(Shallow cracks, limited oil retention) |
| Wear Resistance | ★★★☆☆(Pore edges prone to spalling) | ★★★★☆(Uniform structure, good wear resistance) | ★★★★★(Dense layer, stress-relieving cracks) |
| Anti-Galling Property | ★★★★☆ | ★★★★☆ | ★★★★★ |
| Load Bearing Capacity | ★★☆☆☆(Pores act as stress concentrators) | ★★★☆☆ | ★★★★☆(Micro-cracks disperse stress) |
| Suitable Speed Range | Low speed, heavy load | Medium to high speed | High speed, high load |
Disadvantages of Hard Chrome Plating
- Low Efficiency: The electroplating process is slow, causing to low deposition efficiency(typically only 10%–25%).
- Micro-cracking: Cracks may lead to pitting corrosion.
- Risk of Hydrogen Embrittlement: High-strength steel components require rigorous post-plating hydrogen removal treatment.
3.2. Classification of Chrome Plating on Plating Solution
Hexavalent Chrome Plating
Hexavalent chrome plating is a traditional chromium plating process that has the longest history. Its plating bath typically contains chromic anhydride(CrO₃) as the main ingredient.
- Key Features of Hexavalent Chrome Plating
① Bath Composition: Chromic acid formed by dissolving chromic anhydride in water and catalyst(typically sulfate ions).
② Cathode Reaction: During the electroplating process, hexavalent chromium ions (Cr⁶⁺) in the solution are reduced at the cathode surface to deposit metallic chromium (Cr⁰).
③ Mature Technology: In use for over 80 years. The process is mature and easy to operate.
④ Bright Appearance: Hexavalent chromium plating is silvery white with highly bright gloss and excellent mirror finish.
⑤ Very Low Current Efficiency: Only 10–15% of electrical energy is used for chromium deposition, while most energy is consumed for hydrogen gas evolution.
Advantages of Hexavalent Chrome Plating
① High hardness (800–1000 HV).
② Excellent wear and corrosion resistance.
③ Stable bath chemistry with relatively simple maintenance.
④ Thicker Coating
Disadvantages of Hexavalent Chrome Plating
① High Toxicity: Hexavalent chromium (Cr⁶⁺) is a recognized potent carcinogen, posing severe damage to human health and the environment.
② Strict Environmental Regulations: Globally restricted or banned under regulations such as the EU’s RoHS, REACH, and ELV directives.
③ High Costs for Disposal: The wastewater and exhaust gases generated during production require expensive and complex disposal to meet discharge standards.
Trivalent Chrome Plating
Developed as a replacement for hexavalent chromium plating, trivalent chromium plating has become the mainstream choice for decorative applications.
Key Features of Trivalent Chrome Plating
① Bath Composition: Trivalent chromium salts such as chromium chloride or chromium sulfate, complexing agents, conductive salts, and additives.
② Cathode Reaction: Trivalent chromium ions (Cr³⁺) are directly reduced at the cathode to deposit metallic chromium (Cr⁰).
③Environmental Advantage: Toxicity of trivalent chromium is approximately 1% that of hexavalent chromium, making it significantly more environmentally friendly.
④ Gentle Appearance: Early trivalent chromium coating is bluish-white with gentle gloss. But recent advancements in additives have enabled them to closely match the appearance of hexavalent chromium.
Advantages of Trivalent Chrome Plating
① Environmentally Friendliness: Trivalent chrome coating is the primary alternative to hexavalent chromium plating, compliant with international environmental regulations.
② Excellent Dispersibility: Trivalent chromium coating can be deposited more uniform, especially suitable for complex geometries.
③ Higher Current Efficiency: Up to 25%, offering significant energy savings.
Disadvantages of Trivalent Chrome Plating
① Lower coating hardness (500–700 HV), unsuitable for conditions requiring high wear resistance.
② Limited achievable thickness
③ The bath is sensitive to metallic impurities, requiring more stringent maintenance and control.
Divalent Chrome Plating
Divalent chromium plating is developed to overcome certain limitations of trivalent chromium plating.
Key Features of Divalent Chrome Plating
① Bath Composition: Chloride-based solutions containing divalent chromium ions (Cr²⁺). Cr²⁺ is highly unstable and must be stabilized within specially formulated plating baths.
② Cathode Reaction: Divalent chromium ions (Cr²⁺) are directly reduced at the cathode to deposit metallic chromium (Cr⁰).
③ Cutting-Edge Technology: Currently a major focus of research and development in the chromium plating field.
Advantages of Divalent Chrome Plating
① More Environmentally Friendly: Divalent chromium exhibits even lower toxicity than trivalent chromium.
② Very High Current Efficiency: More than 60%, offering exceptional energy savings.
③ High Deposition Rate: The production efficiency is much higher.
Disadvantages of Divalent Chrome Plating
① Immature Technology: Cr²⁺ ions in the bath are highly easy to become Cr³⁺ by oxidation. It is still a challenge to make it stable.
② High Cost: The bath formulations are complex and expensive to maintain.
③ Limited Application: Divalent chrome plating remains primarily in the laboratory R&D now, not yet widely commercialized.
Table of Comparison: Hexavalent VS Trivalent VS Divalent Chrome Plating
| Feature | Hexavalent Chrome Plating | Trivalent Chrome Plating | Divalent Chrome Plating |
|---|---|---|---|
| Chromium Ion | Cr⁶⁺ | Cr³⁺ | Cr²⁺ |
| Toxicity | High(Carcinogenic) | Low | Extremely Low |
| Environmental Friendliness | Very Poor, Strictly Restricted | Good, Mainstream Replacement | Excellent, Future Direction |
| Hardness | High (800-1000 HV) | Medium (500-700 HV) | —— |
| Current Efficiency | Very Low (10-15%) | Medium (~25%) | Very High (>60%) |
| Coating Color | Bright White | Gentle Bluish-White | —— |
| Coverage | Good | Better | —— |
| Thickness | Able to be very thick | Thin (<1μm) | —— |
| Technology | Very Mature | Less Mature | R&D Phase, Immature |
3.3. Classification of Chrome Plating on Coating Structure
Thin Dense Chrome Plating
Thin Dense Chrome Plating is an electroplated chromium process with excellent performance such as thinner and denser coating, superior adhesion, and enhanced corrosion and wear resistance.
By using specialized chromium baths, this electroplating process can deposit thinner coating. And the high density is due to strict control of plating parameter such as slightly lower temperature.
Thin dense chromium plating is widely used in industries with extremely demanding surface performance requirements, such as aerospace, automotive, tooling and molding, hydraulic systems, and defense.
- Key Features of Thin Dense Chrome Plating
① Extremely Thin Coating: The thickness of thin dense chrome coating typically ranges from 2.5 to 25 micrometers, substantially thinner than conventional hard chrome coating(typically 25–250 micrometers).
② Dense Microstructure: The coating structure is dense and without micro cracks compared with traditional hard chrome coating. Its dense microstructure and extremely low porosity can effectively block the penetration of corrosive media.
③ Low Friction Coefficient: The friction coefficient of thin dense chrome plating can be as low as 0.09, making the coating significantly self-lubricating. With plating of thin dense chrome coating, the friction and wear can be beneficially reduced and it can also prevent galling or seizing between components.
- Advantages of Thin Dense Chrome Plating
① Excellent Corrosion Resistance: Due to its dense structure and minimal micro-cracks, thin dense chrome plating exhibits significantly improved corrosion resistance. In salt spray testing such as ASTM B117, thin dense chrome coating can withstand over 500 hours without base metal corrosion, which far outperform traditional hard chrome coating.
② Outstanding Hardness and Wear Resistance: The hardness of thin dense chrome plating is as much as that of hard chrome plating(800–1000 HV). Meanwhile, combined with uniformly dense microstructure and low friction coefficient, thin dense chrome plating provides excellent resistance to abrasive and adhesive wear.
③ Strong Adhesion: The coating exhibits good bonding strength with the plated metal, enabling it to withstand significant mechanical stress without delamination or spalling.
④ Minimal Influence on Dimension: Thin dense chrome plating is suitable for precision parts that require surface enhancement but has tight dimensional tolerance.
- Disadvantages of Thin-Dense Chrome Plating
① High Cost: The cost of plating material is high due to specialized solution. And the process is complex for high precision, making the cost higher too.
② Limited Thickness: Although thin dense chrome coating is dense and wear-resistant, it is too thin to withstand conditions with high-level and long-term abrasion.
③ Difficult to Local Replating: Once thin dense coating has been damaged or worn, it is difficult to locally repair. Typically, it is required to strip off the entire coating and then replate. But the stripping process might damage the part.
Flash Chrome Plating
Flash Chrome Plating makes the coating highly glossy and mirror-like. And flash chrome coating is extremely thin. It is also mainly used for decoration, but even thinner than decorative chrome plating.
- Features of Flash Chrome Plating
① Extremely Thin Coating: The thickness is about 0.05-0.5 μm.
② Highly Reflective Gloss: With dense and fine-grained structure, the coating can deliver an excellent mirror-bright finish.
③ Short Plating Process: The coating is so thin that the part can be coated much quickly.
- Advantages of Flash Chrome Plating
① Appealing Appearance: Flash chrome plating can provide smooth and shiny finish for the part.
② Minimal Impact on Dimension: Since the coating is extremely thin, it has minimal impact on the part’s dimension.
③ Minimal Impact on Conductivity: Since the coating is extremely thin and uniformly complete, it has minimal impact on the conductivity for electric connectors etc.
④ Cost-effective: The coating can be plated quickly, making it suitable for components with certainly low requirements in batch.
- Disadvantages of Flash Chrome Plating
① Relaying on Base Layer: It is usually required to plate a bright nickel plating as the base layer for flash chrome coating to improve gloss and durability.
② High Requirements for Base Layer: Because the surface defects of base layer cannot be concealed by the thin coating, it must keep highly smooth and finish.
③ Limited Durability and Corrosion Resistance.
Satin Chrome Plating
Different from the shiny and polished look of traditional chrome plating, satin chrome plating is matte or semi-matte and non-reflective.
The plating process typically uses standard chromium bath but it is required to brush the surface before plating to make it with soft satin texture.
And satin chrome plating is preferred by parts opt to subtle and elegant finish such as car parts, door handles, and kitchen utensils etc.
- Features of Satin Chrome Plating
① The surface exhibits a soft silvery-gray tone with a subtle diffuse reflection.
② There are visible brush marks on the coating surface.
- Advantages of Satin Chrome Plating
① With premium and understated finish, satin chrome plating is suitable for modern design.
② Satin chrome coating can effectively conceal fingerprints and fine scratches, making it resistant to dirt.
③ The surface is finely textured and silky smooth, making its touching feel more comfortable than that of highly polished surfaces.
④ The coating has moderate resistance to wear and corrosion.
- Disadvantages of Satin Chrome Plating
① Although the coating can hide fingerprints well, it would be slightly difficult to clean stubborn stains from its micro-textured surface.
② Its durability is lower than traditional hard chrome.
③ It requires specific surface preparation to keep consistency.
Black Chrome Plating
Black chrome plating typically appears black or dark gray. It is not a single chrome coating but is combined with a black compound layer.
The coating is commonly produced by electrochemical method, during which the part plated with chrome coating would be immersed in a specialized plating bath. Then a thin film of chromium oxide would deposit on the surface, which is primarily composited with Cr₂O₃・xH₂O.
- Features of Black Chrome Plating
① The coating shows a matte dark finish with distinct metallic luster from conventional chrome plating. Its color is stable and highly resistant to fading.
② The black chromium coating is not pure chromium. Instead, it consists of chrome, chromium oxides, and fine chromium particles. Such a compound is the key for its black appearance.
- Advantages of Black Chrome Plating
① The matte black appearance is understated and elegant.
② The coating can effectively absorbs glare, making it suitable for optional instruments, camera lens barrels, and high-end automative interior components where reflections must be minimized.
③ The coating has moderate wear and corrosion resistance.
④ The coating is highly resistant to heat without discoloration or deformation.
- Disadvantages of Black Chrome Plating
① The process of black chrome plating is more complex than that of traditional chrome plating, making its cost higher.
② The color would vary based on plating parameters, which would affect consistency.
4. What Materials Can be Plated With Chrome?
Chrome coating can be plated on various materials. The common materials are as follows.
Carbon Steel:
It is the most common substrate for chrome plating. Common applications include automotive components, hardware tools, and general mechanical parts.
Stainless Steel:
Chrome coatingon stainless steel is typically performed to enhance decorative appeal or surface hardness. Special activation is required to ensure adequate adhesion for chromium plating.
Copper and Copper Alloys:
Pure copper offers excellent electrical conductivity, and it is usually polished prior to plating for a mirror-like finish after chrome deposition. Brass plated with decorative chrome plating is widely used for faucets, door handles, bathroom fixtures, and lighting hardware.
Brass itself can easily be polished to be highly bright, and when plated with chromium, it delivers a brilliant and luxurious appearance. Bronze is used for special industrial components or artistic works.
Aluminum and Aluminum Alloys:
Aluminum is highly reactiveand naturally forms a passive oxide layer, which results in poor adhesion for direct plating. Therefore, it is required to be specially treated. Electroless nickel plating is usually performed before chrome plating on aluminum.
Zinc Die-Cast:
Directly plating chrome on zinc die-cast parts would cause defects such as pitting and blistering. Therefore, meticulous surface polishing and multiple undercoating, typically involvinga copper plating followed by a bright nickel plating, are required before the chrome plating. And it is primarily used for decorative electroplating on fashion accessories, zippers, small bathroom fittings, and similar hardware.
Plastic(ABS/SLA Parts):
Plastic plated with chromecoating, particularly ABS, is widely used for automotive grilles, logo badges, faucet handles, and many other decorative components. 
Since non-metal materials are inherently non-conductive, they cannot be electroplated directly, and thus must be specially treated to be conductive first.
Before chrome electroplating, it is required to etch the plastic part to create a microscopically rough surface first and perform activation to absorb catalytic metal particles.
Then, to make the part conductive, electroless plating of copper or nickel should be performed. Finally, the chrome electroplating process can be performed.
Below is an SLA-printed part made in our factory with chrome electroplating, which creates a bright and shiny surface.

5. What are the Common Applications of Chrome Plating?
Automotive Industry: Chrome plating is commonly used on car parts such as engine components, bumpers, exhaust pipes, wheels, and rocker panelsfor better durability and polished appearance.
Industrial Equipment: Chrome coating can be used on machine components such as cylinders, pistons, and hydraulic rodsto enhance abrasion resistance and service life. In addition, chrome plating can also be applied to molds and dies for less abrasion and better part release.
Aerospace: Chrome plating is usually used on aircraft components like landing gear and turbine blades for less friction and abrasion due to the coating’s smooth surface.
Consumer Good: Decorative chrome plating is popularly used on kitchen utensils and fixtures like faucets, handles, and kitchen toolsfor appealing finish and basic corrosion resistance. Additionally, household items such as door handles and furniture accents would often be plated with chrome coating for specific appearance.
Medical Devices: Due to excellent corrosion resistance and durability, chrome plating would be used on surgical and dental instruments for easier sterilization and better hygiene.
Heavy Machinery: Chrome plating can provide excellent corrosion and abrasion resistance for construction equipment and agricultural tools that are often exposed to harsh environment.
Marine Applications: As we all know, parts exposed to marine environment would experienceharsh saltwater corrosion and brushing. Therefore, hard chrome plating would usually be applied to boat fittings and propeller shafts for longer service life.
Oil and Gas Industry: Chrome plating can be used to provide effective protection for pumps, valves and pipeline fittings that are exposed to constantly long-term harsh chemicals, heat and abrasion.
6. What are the Advantages of Chrome Plating?
Excellent Corrosion Resistance:
This is one of the most crucial advantages of chrome plating. With a chromecoating plated, rust and oxidation would be effectively prevented from the part exposed to harsh environments.
Good Hardness:
Chrome plating, especiallyhard chrome plating, can enhance the hardness of the base material to a certainly high level. Therefore, chrome plating is popular to industrial equipment and components that always withstand constant wear.
Decorative Appearance:
Decorative chrome plating can provide parts such as car components, kitchen utensils, and various home fixtureswith a smoothly polished and brightly mirror-like finish. What’s more, chrome plating can also provide specific appeals such as black, satin, or matte appearances.
Longer Durability:
Chrome coating is durable and wear-resistant, especially hard chrome plating. With smooth surface, chrome plating can significantly reduce wear and tear. Therefore, chrome plating is popularly applied to equipment working in high-stress conditions, likehydraulic cylinders and engine parts. And thicker coating means higher hardness, thus better abrasion resistance.
Easy to Clean and Maintain:
Since the chrome coatingis smooth, it is easy to clean parts with chrome coating plated on, which makes chrome plating widely used on medical equipment, kitchenware, and household items.
Custom Thickness:
The thickness of chrome plating is certainly easy to adjust. If chrome plating is mainly used for decoration, the coating can be plated thin. While if mainly for excellent corrosion resistance and longer durability, the coating can be plated as thick as required.
Restoration and Repair:
Chromium coating can be applied to restore worn parts to specific sizes and improve theirsurface performance.
7. What are the Common Problems with Chrome Plating and Their Causes?
Poor Adhesion(Peeling and Flaking)
Causes:
① Inadequate surface cleaning of oil, grease, or oxide scale
② Insufficient activation or exhausted activation solution
③ Improper current density (too high or too low)
④ Poor control of bath temperature or composition
⑤ Substrate issues like high carbon content or inclusions
Hazy, Milky, or Dull Appearance
Causes: ① High levels of impurities in the bath such as Fe³⁺, Cu²⁺, Cl⁻
② Too low or too high bath temperature
③ Incorrect current density
④ Imbalanced ratio of sulfate to chromic acid
⑤ Delayed rinsing during post-process or poor rinse water quality
Rough Surace with Pitting or Pinholes
Causes:
① Solid particulate contamination in the bath like dust and anode sludge
② Unrepaired surface defects such as scratches and pits of the part
③ Inadequate filtration of the plating solution
④ Hydrogen bubbles adhering to the part and not being dislodged
⑤ Insufficient wetting of the part before immersion
Uneven Thickness
Causes:
① Uneven current distribution for complex parts
② Poor rack or fixture design
③ Insufficient cathode movement or solution agitation
④ Poor bath conductivity or low solution level
Cracking
Causes:
① Excessive internal stress
② Excessive thickness
③ Excessively low bath temperature or high current density
④ Improper heat treatment to the part
⑤ Delayed or omitted hydrogen relief
Missed Plating
Causes:
① Local electrical insulation for poor contact at rack
② Residual oil, oxide film, or passivation layer on the surface
③ Local anomalies of bath concentration or temperature
④ Power interruption or poor electrical contact
Yellowing or Discoloration
Causes:
① Incomplete rinsing after electroplating, leaving residual chromic acid
② Delayed drying, leading to surface oxidation
③ Contaminated by impurities in baths
④ Oxidated by excessive drying temperature
Environmental and Safety Concerns
Causes:
① Toxic emissions and wastewater generated by hexavalent chromium plating
② Cr⁶⁺ is a known carcinogen, posing serious health and environmental damages
8. How to Maintain Chrome Plating?
Maintenance of chrome-plated parts focuses on preventing oxidation/corrosion and physical scratches. Maintenance strategies should be tailored to their specific usage scenarios. Below is a detailed maintenance guide.
For Outdoor Daily Items:
These parts endure constant sun, wind, rain, and road debris, requiring emphasis on oxidation prevention and impact protection.
① Weekly Basic Cleaning:
Firstly, rinse the part thoroughly with clean water to remove dust and sand. And note that dry rub is not allowed, which would cause scratches.
Secondly, gently wipe the part with a soft sponge or microfiber cloth dampened with neutral car wash soap (pH 6–8). And note to pay special attention to positions where oil, insect residue, or grime accumulates.
Finally, completely rinse the part with clean water and immediately dry it with a clean absorbent towel since residual moisture would accelerate oxidation.
② Monthly Deep Maintenance:
Firstly, evenly apply chrome-specific protectant such as automotive chrome wax or silicone-based sealant over the surface after completely cleaning and drying.
Secondly, let it rests for 5–10 minutes, and then buff it shine with a clean cloth
This would form a film that shields against rain, UV rays, and environmental pollutants.
- For Indoor Items: These parts are mainly affected by dust, fingerprints, sweat, and light oil residues.
① Cleaning Every 2-3 Weeks:
Firstly, wipe dust away with a dry microfiber cloth. Note to do not use rough rags that cause micro-scratches.
Secondly, gently wipe the part by a cloth dampened with diluted neutral dish soap if there are light oil stains on it. And then dry it immediately with a clean cloth.
② Maintaining Every 3 Months:
For rarely used items plated with chrome coating, apply a tiny amount of petroleum jelly (Vaseline) or baby oil taken by a cotton swab. This creates a thin protective barrier against oxidation.
For Parts with White Spots on Surface Caused by Light Oxidation
Firstly, dampen the oxidized area with water and apply a small amount of toothpaste to the cloth. Note that the toothpaste must be regular one with mild abrasives but not gel types.
Secondly, gently rub the area along small clockwise circles with light pressure for 1–2 minutes until the white spot fades.
Finally, rinse the part thoroughly to remove toothpaste residue and dry it completely. And apply chrome protectant afterward to prevent recurrence.
For Parts with Stubborn Stains
If the stains are bird droppings or tree sap, soak a cloth or cotton swab in warm water, and then place it over the stains for 5 minutes to soften them. Finally, gently wipe the part with neutral cleaner.
If the stains are heavy grease, dab a cotton swab with a small amount of 75% isopropyl alcohol or gasoline, and then gently clean the stains spot by spot. Note to avoid large applying in case of dissolving the protective film. And then rinse the part with water and dry it thoroughly.
For Parts with Minor Scratches
Firstly, clean the scratched area to remove dust.
Secondly, apply a small amount of wax to the cloth and gently buff the scratched area along small clockwise circle with evenly light pressure and slow motion for 30–60 seconds.
Finally, wipe off residue with a clean cloth. If the scratch remains visible, repeat once or twice.
9. How to Remove Chrome Plating?
There are three main methods for chrome plating removal, including chemical stripping, mechanical removal, and reverse electroplating. It is required to choose the proper method according to the base material and specific properties of these methods.
Chemical Stripping
① Commonly Used Solutions
Table of Commonly Used Solutions for Chemical Stripping
| Solution | Composition | Temperature | Immersion Time | Suitable Material |
|---|---|---|---|---|
| Dilute Hydrochloric Acid | Hydrochloric acid (37% industrial grade) : Water = 1:1 to 1:3 | Room Temperature to 50°C | 10–30 minutes | Steel (not suitable for copper) |
| Dilute Sulfuric Acid | Sulfuric acid (98% industrial grade) : Water = 1:5 to 1:8 | 40–60°C | 20–40 minutes | Steel, Stainless steel |
| Chromic Acid–Sulfuric Acid | Chromic anhydride (CrO₃) 100 g/L + Sulfuric acid 50 mL/L | Room Temperature | 30–60 minutes | Copper, Copper alloys |
② Procedure
Firstly, wipe the part with alcohol or acetone to remove oil, grease, and dust.
Secondly, prepare the stripping solution according to the specified formulation in an acid-resistant container like polypropylene plastic bucket or ceramic tank. And then immerse the whole part in the solution and gently agitate periodically.
Thirdly, observe the dissolving condition of the chrome coating. It will gradually flake off, and the solution will turn bluish-green or black.
Fourthly, as soon as the base metal becomes visible (when the steel appears silvery-gray or the copper appears reddish-purple), immediately pick the part out and rinse it thoroughly with clean water 3–5 times to eliminate all residual acid.
Finally, immerse the part in a mild alkaline solution for 5 minutes to neutralize any remaining acid. And then rinse it again with clean water, followed with drying by a clean cloth or hot air.
③ Key Precautions
- Always wear acid-resistant gloves, safety goggles, and a respirator or fume mask and work in a fume hoodduring operation.
- When preparing acid solutions, always add acid slowly to water. Adding water to concentrated acid wouldcause violent boiling and splashing.
- Never mix different acidstogether.
- Neutralize all waste solutions before disposal.
Mechanical Removal
① Tools and Procedure
Table of Tools and Procedure for Mechanical Removal
| Tool | Characteristics | Procedure |
|---|---|---|
| Sandpaper (800–2000 grit) | Coarse grit for plating removal with fine grit for polish | 1. Lightly sand the chrome layer along one direction by 800-grit paper until the base metal is exposed. 2. Switch to 1500–2000-grit paper to polish the base metal and remove sanding marks. 3. Wipe the surface with alcohol to remove dust. |
| Angle Grinder with Flap Wheel | Suitable for large areas or thick chrome layers | 1. Install a 120-grit flap wheel and operate at low speed (≤1500 RPM) to gently contact the chrome surface. 2. Hold the grinder at a 15° angle to the part and move slowly to avoid local overheat. 3. Switch to a 240-grit flap wheel for fine grinding, followed by buffing with a cloth wheel. |
| Ultrasonic Cleaner | Ideal for tiny crevices and precision components | 1. Fill the tank with water and a neutral degreaser, then submerge the part in it. 2. Activate the ultrasonic cleaner (500–800 W), combined with light manual abrasion if needed. The ultrasonic vibrations help dislodge loose chrome plating. 3. Repeat as necessary. |
② Key Precautions
- Sand or grind the part at a consistent speed with light pressure to avoid scratches or deformation, especially critical for soft metals like aluminum alloys.
- For grinding of large surface, work intermittently to prevent the part from overheat.
- It is recommended to use ultrasonic cleaningfor precise components.
Reverse Electroplating
① Procedure
Firstly, fill the acid-resistant plastic container with electrolyte.
Secondly, connect the part to the positive terminal as the anode, and connect a pure copper plate or graphite plate to the negative terminal as the cathode. Note to maintain a distance of 5–10 cm between the electrodes.
Thirdly, adjust the voltage to 5–12 V (higher voltage for thicker chrome layers) and the current density to 1–3 A/dm².
Fourthly, apply current and observe the stripping condition. Bubbles will form on the anode surface, and the chrome coating will gradually dissolve. Note to turn off the power every 5 minutes to inspect the progress and continue until the chrome layer is completely removed.
Finally, turn off the power and pick the part out if the coating is completely stripped off. And then rinse the part thoroughly with clean water, followed by drying.
② Key Precautions
- Prepare the electrolyte with pure water without impurities that could interfere with the electrolysis process.
- Always wear insulated gloves during operation to prevent electric shock.
- If the base metal is aluminum, add 0.5% corrosion inhibitorinto the electrolyte to prevent dissolution of the aluminum.
10. Comparison of Chrome Plating with Other Finishing Techniques
Chrome Plating vs. Nickel Plating:
Both of them can provide good corrosion resistance and a shiny finish. But compared with nickel plating, chrome plating, especially hard chrome plating, has better durability and wear resistance.
Nickel plating, especially electroless nickel plating, can offer a smoother finish because of more uniform coverage for complex parts.
Chrome Plating vs. Powder Coating:
Different from chrome plating, powder coating is a dry finishing technique that creates a thick protective layer on the part’s surface.
However, its hardness and abrasion resistance are worse than those of chrome plating. Powder coating stands out in its rich color variety and good environmental friendliness. It does not emit volatile organic compounds (VOCs).
Chrome Plating vs. Anodizing:
Compared with chrome plating, anodizing is particularly applied to aluminum. It would provide aluminum parts with a thin oxidized coating to enhance corrosion resistance.
What’s more, anodizing coating can be colored and show a matte finish, which is different from the shiny appearance of chrome plating. Its hardness and wear resistance are also worse than those of chrome plating.
Chrome Plating vs. Zinc Plating:
Compared with chrome plating, zinc plating provides sacrificialprotection especially for steel components. It can enhance the corrosion and abrasion resistance of the base material.
But chrome plating can provide better hardness and durability. Chrome plating is much more suitable for parts exposed to harsh environments. In addition, chrome plating provides smoother surface.
Chrome Plating vs. PVD:
The hardness of PVD coating is closely same as that of chrome plating. But since PVD coating is significantly dense while chrome plating is micro cracked, both the corrosion and wear resistance of the former is better than that of the letter.
What’s more, compared with chrome plating, PVD coating can be more richly colored.
| Feature | Chrome Plating | Nickel Plating | Powder Coating | Anodizing | Zinc Plating | PVD |
|---|---|---|---|---|---|---|
| Hardness (HV) | 800–1000 | 150–250(electroplating) 500–1000 (electroless plating) | 20–100 | 300–600 | 70–150 | 1500–3000 |
| Wear Resistance | ★★★★★ | ★★★☆ (electroplating) ★★★★ (electroless plating) | ★★ | ★★★☆ | ★★ | ★★★★★ |
| Corrosion Resistance | ★★★★☆ | ★★★★ | ★★★★ | ★★★★ | ★★★☆ | ★★★★★ |
| Environmental Friendliness | ★ | ★★☆ | ★★★ (solvent-based) ★★★★ (powder) | ★★★★ | ★★★☆ | ★★★★★ |
| Decorative Appearance | Excellent | Good | Excellent | Excellent | Fair | Excellent |
| Cost | High | Medium–High | Medium | Medium | Low | High |
| Substrate Compatibility | Steel, copper, etc. | Most metals | Almost all materials | Aluminum, magnesium, titanium | Ferrous metals | Metals, ceramics |
11. Conclusion
There are various finish techniques for different applications. Chrome plating standouts for its excellent hardness, corrosion resistance, durability and smoothly bright appearance. It is important to know these finish techniques comprehensively and choose the right one for applications. This article has provided a detailed guide of chrome plating. But if you still has question, contact with us freely.
⚠️ SAFETY WARNING: Hexavalent Chromium Health Risks
Hexavalent chromium compounds are known to be:
- Potent Carcinogens – May increase risk of lung cancer
- Highly Toxic Substances – Can damage respiratory system, liver, and kidneys
- Environmental Pollutants – Persistent in environment and bioaccumulative
- Skin Sensitizers – May cause contact dermatitis and skin ulcers
Essential safety measures: Use professional protective equipment, work in well-ventilated areas, comply with local environmental regulations, and dispose of waste properly.
Disclaimer: This content is provided for reference and educational purposes only and does not constitute professional advice. Specific operations should be consulted with qualified engineers, environmental specialists, or relevant professionals, and must comply with local laws, regulations, and safety standards. The author and publisher assume no responsibility for any losses or damages resulting from the use of this information.

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He’s a lifelong learner who loves sharing his expertise.
Other Articles You Might Enjoy

What is Nickel Plating?
Nickel plating coats a thin nickel layer via electroplating or chemical methods. Two main types are Electroplating Nickel (EN) and Electroless Nickel Plating (ENP).

Zinc Plating - Everything You Shall Know
Zinc Plating are classifed into Hot-Dip Galvanizing, Electro Zinc Plating,Mechanical Zinc Plating and Electroless Zinc Plating.

Vapor Polishing: Step by Step Guide for Plastics
Acrylic and polycarbonate CNC-machined parts can achieve high transparency after manual polishing and vapor polishing.

Aluminum Alloy Anodizing-MIL-PRF-8625F
Aluminum anodizing is systematically classified under MIL-PRF-8625F into six distinct Types (I, IB, IC, II, IIB, III) and two Classes (1: non-dyed, 2: dyed).


