Table of Contents
Both electropolishing and passivation are common finishing techniques mainly used on stainless steels. They are both applied to improve the surface appearance and performance, but in different ways.
Electropolishing is a type of electrochemical finishing method, whereas passivation is a chemical process that does not involve electrical current.
Apart from this basic distinction, there are still various differences between electropolishing and passivation. It is also important to know them well and choose the proper one for metal products.
Key takeaways:
- Electropolishing is mainly for smoothing, brightening, and removing micro-defects, while passivation focuses on removing free iron and enhancing corrosion resistance through a thin oxide film.
- Electropolishing is an electrochemical removal process that dissolves microscopic peaks to produce a mirror-like finish.
- Passivation is a non-dimensional chemical treatment, ideal when you need corrosion protection without altering part size.
1. What is Electropolishing?
Electropolishing is a type of electrochemical finishing technique that is used to remove a certain amount of material from the surface and make it smoother and brighter.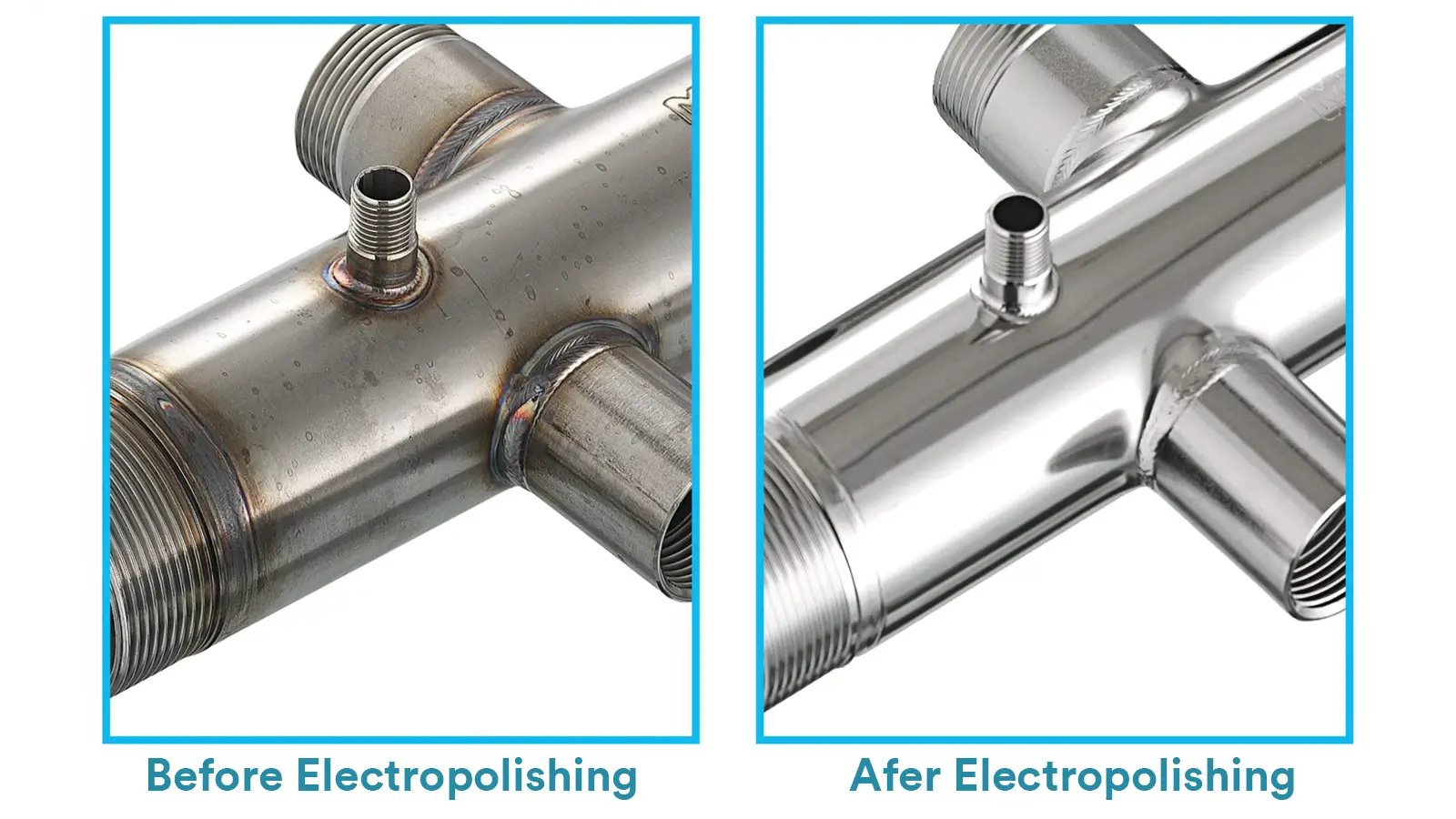
The process can meanwhile enhance the surface’s corrosion resistance, as it reduces micro imperfections and helps enrich the chromium concentration.
This would shut down the chance for corrosive items to damage the surface and form a thin passivated layer, providing strong protection.
The working principle depends on the different distribution of electrical density on the surface. During the electropolishing process, the part is immersed in the electrolyte solution and acts as the anode, while a piece of lead serves as the cathode.
Once the direct current is applied to the electrolyte bath, an oxidation reaction would occur on the part’s surface, during which the metal atoms will lose their electrons and become ions in the electrolyte.
But such a dissolution is not uniformly distributed on the surface due to its roughness. The current density would be higher at the protrusions, so metal ions in those areas would dissolve more rapidly.
In contrast, lower current density in the recesses would lead to slower dissolution. Over time, the surface will gradually become smooth.
For a better explanation, you can compare the surface to a landscape of rolling hills, with electric current flowing across it like water. On the higher hills, the flow would move faster and erode the hills more quickly. While that in the valleys would be slower and lead to weaker erosion.
For more information about electropolishing, please visit our blog:
2. What is Passivation?
Passivation in chemics refers to the phenomenon in which certain metals such as iron, aluminum, and stainless steel will react with oxygen or other oxidizing agents in the environment to form a dense, stable, and inert oxide protective film on their surface.
Due to its passivated state with lower chemical reactivity, the film can reduce the metal’s tendency toward further corrosion.

In industry, passivation is exactly a surface finishing technique that is applied to improve the passive oxide layer on the metal and thus to enhance the surface’s corrosion resistance.
Although the metal itself can form a passivated film on the surface inherently, the film would usually not be complete and even enough.
What’s worse, there might be certain amounts of iron particles embedded in the surface during mechanical processing.
They would impair the surface’s natural passivation film and make it more likely to be eroded. Therefore, passivation is needed for these metal parts.
Passivation is commonly a chemical process that does not involve the use of electrical current. It primarily depends on the passivation solution, which mainly includes citric acid solutions and nitric acid solutions.
When stainless steel is immersed in the passivation solution, the free iron is primarily oxidized and dissolved, while the chromium within the part combines with oxygen to form a passivation film.
With excellent density and stability, the film can effectively protect the surface from various corrosive agents, both physically and chemically.
3. What is the Process of Electropolishing?
- Pre-process: It is the most crucial step for electropolishing, to make the surface clean enough. If there is any imperfection left on the surface, it might exhibit spots, streaks, or uneven gloss after polishing.
- Degreasing: Remove oil, fingerprints, and other organic contaminants by an alkaline cleaner or solvent.
- Pickling: Remove oxide scale or rustusing a solution with nitric acid and hydrofluoric acid.
- Rinsing: Rinse the part with deionized water to prevent the electrolyte from
- Drying: Dry the part completely to ensure the surface free of water, thus to avoid local short circuits.
- Fixturing and Racking: Secure the part onto the rack to ensure uniform current distribution. The rack should have good electrical conductivity and corrosion resistance. And the contact area between the rack and the part should be appropriate to ensure even current distribution.
- Electropolishing: Immerse the rack with the part and the cathode into the electrolyte Then apply direct current in the tank. During the polishing process, it is important to properly control the voltage and density of the direct current as well as the electrolyte temperature and polishing time.
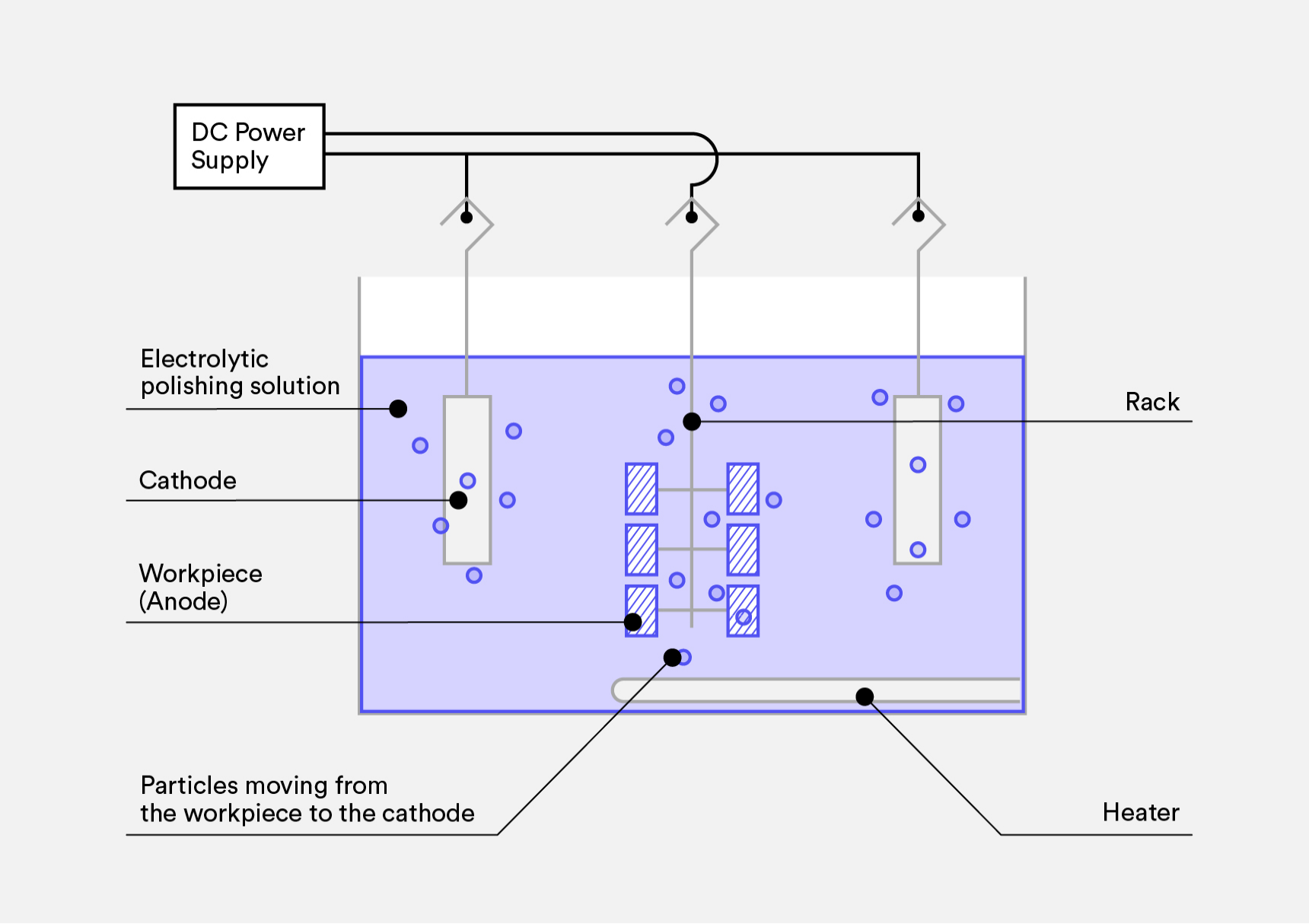
As the part is electropolished, anodic dissolution would occur on the surface. With the microscopic protrusions rapidly dissolved, the surface would gradually be smooth and glossy.
In addition, note to properly stir and circulate the electrolyte bath for uniform temperature and concentration.
- Post-process: After electropolishing, the surface must be completely cleaned from any residual solution.
- Rinsing: Rinse the part immediately and rapidly with a large amount of flowing deionizedwater to stop reaction.
- Neutralizing: Neutralize any residual acid on the surface by a mild alkali like a sodium bicarbonate solution.
- Passivation (optional): Passivate the part for better corrosion resistance.
- Drying: Completely dry the part by hot air or a centrifuge to keep it from any moisture.
4. What is the Process of Passivation?
- Pre-process: Before passivation, it should keep the surface totally clean for uniformformation of the passivated layer.
- Degreasing: Immerse the part in an alkaline degreasing solution to thoroughly remove oils, lubricants, fingerprints, and other organic contaminants from the surface.
- Rinsing: Rinse the part completelywith flowing water to clean residual degreasing bath off from the surface.
- Pickling: Immerse the part in a pickling solution to remove oxide scale, rust, free iron or other impurities from the surface. The pickling solutions mainly include nitric acid bath and that mixed with nitric acid and hydrofluoric acid.
The former one is milder for light rust while the later one is suitable for removing heavy oxide scale.
- Rinsing: Rinse the part with flowing water to clean residual pickling solution off.
- Passivation: Immerse the part into the passivation The solution would primarily dissolve the free iron on the surface and make it rich with chromium. Then there would be an extremely thin and dense oxidation film forming on the surface, which is mainly comprised with Cr2O3.
 The passivation solutions mainly include nitric acid solution and citric acid solution. During the passivation process, it is important to concisely control the concentration, temperature, and passivating time.
The passivation solutions mainly include nitric acid solution and citric acid solution. During the passivation process, it is important to concisely control the concentration, temperature, and passivating time.
- Post-process:
- Rising: Rinse the part with flowing water to clean the any residual bath off from the surface.
- Drying: Dry thepart completely by hot air.
5. What are the Pros and Cons of Electropolishing?
5.1. Advantages of Electropolishing
- Improving Surface Smoothness:
Electropolishing can significantly reduce surface roughness to achieve a mirror-like finish. It can eliminate fine scratches and machining marks, making the surface level, smooth, and glossy.
- Removing Micro Burr:
Electropolishing can effectively eliminate burrs on complex components such as gears, meshes, inner holes, and threadswithout damaging delicate or precise structures.
- Enhancing Corrosion Resistance:
Electropolishing can remove residualfree iron and other inclusions from the surface, which can eliminate potential sources of pitting corrosion.
More importantly, there would be a oxide passivation film forming on the surface since iron would be preferentially dissolved during electropolishing process.
- No Mechanical Stress:
Electropolishing is an electrochemical process. Compared with mechanical polishing, electropolishing is not prone to issues such as hardening, mechanical stress, or microcracks caused by cutting force or frictional heat.
Therefore, electropolishing is suitable for thin-walled parts, elastic components, and finemedical instruments that are sensitive to mechanical stress.
- Suitable for Complex Parts:
Compared with mechanical polishing, electropolishing is suitable for complex parts.
The electrolyte can reach complex inner corners, deep holes, cross holes, and fine structures that are difficult to access with mechanical polishing.
Electropolishing can effectivelyremove burrs from all edges and hole openings.
- Excellent Surface Cleanliness:
The surface after electropolishing is smooth and clean without pores, making it difficult for contaminants or bacteria to accumulate.
This meets the stringent cleanliness standards required in industries such as food processing, pharmaceuticals, and medical equipment.
5.2. Disadvantages of Electropolishing
- High Cost:
Electropolishing needs specific power supplies, corrosion-resistant electrolytic tanks, fixtures, and costly electrolytes, all of which require certainly high cost.
In addition, the electrolyte must be regularly maintained or refreshed, whichincreases polishing cost too.
- Strict Requirement for Initial Surface:
Electropolishing is primarilyused to improve the surface’s smoothness on microscopic level.
For parts with excessively rough surface, electropolishing can only provide slow polishing rate and poor effect.
Heavy oxide scales or rust need to be mechanically or chemically polished before electropolishing. And improper processing would lead to staining or slight corrosion.
- Limited Uniformity:
For parts with extremely complex shapes or deep recesses, the distribution of current density may be uneven, thus leading to inconsistentpolishing effects across the surface.
To ensure uniform current distribution, complex parts often require specially designed and manufactured custom fixtures.
- Limitation on Materials and Shapes:
Electropolishing is only suitable for electrically conductive materials. And it is unsuitablefor carbon steel.
- Environmental and Safety Concerns:
Most electrolytes contain toxic and hazardous substances. It requires to strictly treat the waste liquids and gases generating during the process, which would increase both safety risks and environmental compliance costs.
6. What are the Pros and Cons of Passivation?
6.1. Advantages of Passivation
- Enhancing Corrosion Resistance:
The primary function of passivationexactly lies on that it can completely clean off the free iron within the surface and then form an oxide film to protect the part.
Since the protective layer is extremely thin, dense, and stable, it can effectively isolate the metal substrate from corrosive agents such as water, oxygen, acids, and alkalis.
And carbon steel particles or free iron left on the surface during the mechanical processes such as cutting, welding, and grinding are usually the primary sources of corrosion.
Because they are cleaned from the surface by passivation, the part would not be corroded that easy.
- Preserving Original Dimension and Appearance:
The passivationlayer is typically only a few nanometers () thick. It will not virtually impact the part’s dimensional accuracy, making it ideal for precisely fine components.
Additionally, the passivation film is usually transparent or semi-transparent, so it does not alter the metal’s original color or luster.
Passivation would not change the part’s aesthetic appearance, making it suitable for applications requiring high consistency on appearance.
- Enhancing Self-Healing Ability:
Passivation films can repair by themselves in environments rich with oxygen. If the surface is slightly scratched, the exposed chromium will re-oxidize to form a new protective film.
- Simple Process and Low Cost:
The passivation processes do not require complex equipment, typically only an immersion tank, heating unit, and cleaning system.
Compared with electropolishing and electroplating, passivation cost much lower. Therefore, it allows for batch processing.
- Good Uniformity:
Passivation is a chemical reaction process.
Since passivation solution caneasily reach any surface area of the part, including deep holes, internal cavities and any complex geometries, the passivation film can usually form on the surface uniformly.
Passivation can offer superior uniform coverage compared with mechanical polishing or coating. While compared with electropolishing, passivation does not depend on current density, making the film consistently uniform.
6.2. Disadvantages of Passivation
- Limited Adhesion and Wear Resistance:
Although the passivation film is stable, it is extremely thin and has relatively weak adhesion.
If the part experiences mechanical friction, scratching, or strong impact, the film can be easily damaged.
Once the passivation layer is damaged, it relies on oxygen in the environment to regenerate.
However, in harsh environment or that is short of oxygen, the repair process may be slow or incomplete, which would lead to corrosion.
- Strict Requirement on Initial Surface:
The effectiveness of passivation depends entirely on the thoroughness of prior cleaning, degreasing, and pickling.
If the initialsurface cannot be completely cleaned, the passivation film will not form uniformly or effectively.
What’s more, passivation cannot cover or repair macroscopic defects such as scratches, pits, or rough textures.
For applications with high requirements on aesthetics, mechanical or electro polishing is still necessary beforehand.
- No Improvement on Roughness:
Passivation cannot remove scratches, pits, or burrs.If the surface is much rough, it would still trap contaminants even after passivation, offering only limited improvement in corrosion resistance.
Commonly, it needs to perform mechanical polishing or electropolishing before to achieve optimal effect.
7. Top 5 Differences between Electropolishing and Passivation
Although electropolishing and passivation are both surface finishing technique, mainly used to protect the part’s surface, there are various differences between them. It is important to know their distinctions clearly.
- On Process:
Electropolishing is an electrochemical process that needs to apply currentpower into the electrolyte solution. But passivation is a pure chemical process that does not need electrical current.
- On Function:
The primary functionof electropolishing is to smooth and brighten the surface.
Through anodic dissolution, electropolishing can selectively remove micro imperfections of the metal and then achieve desired smoothness and brightness.
However, passivation is mainly used to enhance the corrosion resistance of the metal surface.
It can remove free iron through chemical reactions and promote the formation of a dense passive film, which can effectively eliminate the primary source of corrosion and strengthen the ability to protect the surface.
- On Appearance:
By removing micro peaks, burrs, cracks and other imperfections on the surface, electropolishing would obviously change the part’s appearance.
The roughness of electropolished parts would be significantly reduced and the surface would become brightly mirror-like.
Compared with electropolishing, passivation would not change the surface’s appearance. It can only clean free iron off from the surface but cannot remove any imperfection.
And the passivation film is usually transparent. Therefore, the roughness and brightness of the surface would be preserved after passivation.
- On Dimension:
Electropolishing has a much larger impact on the part’s dimensions than passivation.
Although the electropolishing’s amount of material removal is certainly small and controllable, it would still reduce the part’s dimension and the dimensional tolerance should be carefully controlled, especially for precisely fine components.
However, passivation would not dissolve any materialof the surface. It would only remove free iron from the surface. Its influence on the dimension is extremely small and does matter nothing.
- On Uniformity:
For complex parts with similarly good cleanliness, passivation would behave better than electropolishing.
Because the surface’s uniformity of electropolishing depends on the current density.
When current flows over the surface, it would naturally concentrate at tips, edges, and protrusions, making these areas polished best.
But the current density of recessed areas, deep holes, and internal corners would be relatively low, leading to slower dissolution and poorer polishing performance.
While passivation can form uniform protective film with consistent thickness and component.
8. When to Choose Electropolishing for CNC Parts?
Electropolishing is commonly applied to CNC parts for below reasons.
- For Corrosion Resistance:
After electropolishing, the part can achievea smooth surface, which can resist the adhesion of corrosive substances.
In addition, a passivation film would form during the electropolishing process, thus providing good corrosion resistance for the components.
Therefore, electropolishing is suitable for medical devices, food equipment, and chemical equipment.
- For Clean Surface:
Electropolishing can significantly reduce the surface’s roughness. It can remove micropores, burrs, and cracks, preventing bacterial accumulation, particle retention, or fluid residue.
Therefore, electropolishing is commonly applied to medical implants, pharmaceutical reactor components, and semiconductor fluid lines.
- For Good Performance:
Electropolishing can improve the smoothness of the CNC parts and eliminate the stress concentrators.
For components of fluid system such as valves, fittings, and microchannels, smooth inner walls would reduce flow resistance, turbulence, and cavitation.
For parts under over circular loading such as springs and aerospace components, less stress concentrators means to longer service life.
- For Bright Appearance:
Electropolishing can make the surface uniformly bright on a whole, without brushings or swirls. It is commonly used for high-end instrument housings and decorative stainless steel.
Conditions Unsuitable for Electropolishing Table
| Condition | Reason |
|---|---|
| Extremely tight dimensional tolerance and no stock allowance | Electropolishing removes material typically 10–50 μm, which may cause out-of-tolerance dimensions. |
| Conductive parts or with dissimilar materials | The electrochemical process may corrode unintended areas. |
| Free-machining stainless steels | Sulfur/selenium inclusions may cause a hazy or spotty surface after polishing. |
9. When to Choose Passivation for CNC Parts?
Passivation is commonly applied to CNC parts for below reasons.
- For Free Iron Removal:
During CNC machining, the cutting tools may embed free iron particles into the stainless steel. These iron particles tend to corrode first in humid or corrosive environments. Passivation would dissolve these free iron contaminants, restoring the stainless steel’s inherent corrosion resistance.
- For High Dimensional Accuracy:
Since passivation would not virtually change the part’s dimension, it is suitable for precisely fine components whose tolerances are small or for finished parts that have already reached theirfinal dimensions.
- For Low Cost:
The passivation process is relatively simple and fast, so it is far more economical than electropolishing for large batches of standard components such as fasteners, flanges, and valve bodies.
- For Corrosion Resistance:
Passivation is mainly used to enhance the part’s corrosion resistance. For parts used in general industrial, indoor atmospheric, or mildly corrosive environments, passivation alone is sufficient to meet ASTM or industry corrosion resistance standards.
Conditions Unsuitable for Passivation Table
| Condition | Reason |
|---|---|
| Obvious burrs, scratches, or roughness | Passivation does not improve surface morphology |
| Severely rusted or oxidized | Passivation cannot remove rust. Acid pickling or mechanical cleaning must be performed first. |
| Highly corrosive environments | Passivation alone may be insufficient. Electropolishing should be combined for optimal performance. |
10. FAQs
10.1. What is the Primary Distinction between Electropolishing and Passivation?
The primary difference between electropolishing and passivation lies on their working principle and purpose. Electropolishing is an electrochemical process used to remove surface imperfections and make it smooth and bright.
While passivation is a chemical process used to make the surface passivated, stable, and resistant to corrosion. Passivation alone cannot improve the part’s cleanliness.
10.2. Which Industries Mostly Require Both Electropolishing and Passivation?
In fields such as medical devices, precision components, aerospace, as well as food and pharmaceutical processing equipment that demand extremely high level of surface cleanliness, smoothness, and corrosion resistance, specifications and technical standards frequently call for both electropolishing and passivation to be performed in sequence. Electropolishing first, and then passivation.
10.3. Which Process is Better for Welds and Heat-tined Areas: Electropolishing or Passivation?
Passivation can not remove rust or heat tint. Electropolishing can dissolve oxide scale and form chromium oxidation film to protect the surface.
10.4. Can Electropolishing Replace Passivation?
Yes. During the electropolishing process, a thin passivated film would usually form on the surface. However, in certain industries such as biomedical and pharmaceutical fields, passivation is needed to further strengthen the surface’s corrosion resistance.

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He’s a lifelong learner who loves sharing his expertise.
Other Articles You Might Enjoy

Electropolishing — Principles, Process, Advantages.
Electropolishing is an electrochemical process that removes microscopic surface peaks from metals, resulting in a mirror-like smooth finish, improved corrosion resistance, and reduced contamination risk.

A Practical Guide to Passivation for CNC Machined Parts 2025
Passivation chemically creates an ultra-thin, dense, and stable oxide film, transforming the metal from an “active” to a “passive” state. This process significantly enhances corrosion resistance without altering the part’s dimensions.

Electropolishing vs Passivation: Learn the Differences
Both electropolishing and passivation are common finishing techniques mainly used on stainless steels. They are both applied to improve the surface appearance and performance, but in different ways.

Chrome Plating VS Nickel Plating
Chrome plating and nickel plating are two common types of finishing techniques. They can both provide enhanced corrosion and abrasion resistance and an appealing appearance. The coatings plated on the part surface can make it more decorative and durable.




