Table of Contents
In recent projects, we’ve had the opportunity to produce a batch of high-quality CNC parts for an international client. The material used was 1020 steel, and the required surface treatment is Phosphatation Fe/Mn grasse/fat phosphating.
While researching this process, we found that detailed and organized resources on this topic were scarce. After visiting multiple phosphating factories in China and conducting extensive research, we have completed this in-depth guide on phosphate conversion coatings.
In this guide, we’ll explore the benefits, processes, classification, and applications of phosphate conversion coating. Let’s start!
1. What is Phosphate Conversion Coating?
Phosphate conversion coating, also known as phosphating, is a surface treatment process that forms an insoluble phosphate protective film on the metal surface through a chemical conversion reaction.
This process typically involves immersing metals (mainly steel, but also aluminum, zinc, etc.) into a phosphating solution, which contains phosphoric acid and phosphates. Alternatively, this process can also be performed using spraying techniques.
The chemical reaction between the metal surface and the phosphating solution creates a dense, insoluble phosphate film (also known as a phosphating coating) on the metal.
Phosphate coatings are highly effective in improving metal corrosion resistance, enhancing the adhesion of subsequent coatings, improving wear resistance, and providing a better base for painting or lubrication.
The core principle of phosphating is a series of chemical reactions that occur when the metal surface comes into contact with the phosphating solution.
For phosphating steel, the metal’s iron is dissolved by the acid in the phosphating solution, releasing Fe²⁺ ions. These ions then combine with phosphate ions (PO₄³⁻) in the solution to form iron phosphate.
The resulting iron phosphate film interacts with metal ions (such as Zn²⁺ or Mn²⁺) from the solution, forming a composite crystalline coating.
Chemical reaction:
3Fe + 2H₃PO₄ → Fe₃(PO₄)₂↓ + 3H₂↑
As the reaction progresses, the local pH of the solution increases, and phosphate salts gradually deposit on the metal surface, forming a crystalline film made of iron phosphate, zinc phosphate, manganese phosphate, and other compounds. The coating adheres strongly to the metal surface.
The resulting phosphate coating is typically composed of phosphate compounds (e.g., zinc phosphate, manganese phosphate, iron phosphate), with a thickness ranging from 0.1 to 20 microns, depending on the type of phosphating and the process parameters.

2. History of Phosphating
The earliest research on phosphating processes were back to the period between 1869 and 1906.
In 1907, Thomas Watts Coslett from the USA was awarded the first patent for iron phosphating. The initial phosphating solution developed was manganese-based.
In 1938, the patent for the zinc-based phosphating solution was got.
During World War II, phosphating was first widely used, particularly in the United States, where it was used for its corrosion-resistant properties and durability in military applications.
3. Advantages of Phosphate Coatings
3.1. Improving Corrosion Resistance
Phosphate coatings significantly enhance the corrosion resistance of metals, offering both physical and chemical benefits.
Physical Stability:The phosphating coating, especially zinc and manganese-based,is dense and serves as a barrier, preventing direct contact between the metal substrate and environmental elements such as air, moisture, and corrosive agents (e.g., salts and oils). It will reduce electrochemical corrosion.
Chemical Stability: Phosphate coatings, primarily made of phosphate compounds, are chemically stable and resistant to reactions with common acids and bases. They can serve as a short-term rust protection coating (e.g., during storage or transportation) and, when combined with oil coatings, offer long-term corrosion resistance.
For instance, car chassis parts treated with phosphating + oil coating can achieve rust protection for more than 6 months in humid environments—much longer than untreated parts, which typically last only 1-2 weeks.
3.2. Improving Adhesion to the Substrate
Phosphate coatings create a rougher surface, improving the adhesion of subsequent coatings like paint or oil.
The microscopic structure in the phosphate film allows coatings such as paints and powder coatings to penetrate and form a mechanical bond. This will significantly enhance the adhesion strength.
It will also prevent peeling, bubbling, and other coating failures—a phenomenon often referred to as the “anchoring effect.”
Additionally, the phosphating process removes contaminants like oxidation, oil, and rust.
It will provide a clean and uniform surface for better coating adhesion.
For example, steel plates treated with phosphating can achieve a 30%-50% increase in paint adhesion, improving impact resistance and weatherability (e.g., extending the lifespan of automotive paint finishes by 5-10 years).
3.3. Enhancing Wear Resistance
Phosphate coatings have moderate hardness and improve the wear resistance of metal components.
Manganese phosphating, in particular, creates a thicker coating than iron phosphating, with hardness reaching over 500HV.
With a low friction coefficient, manganese phosphating is ideal for mechanical parts such as engine gears and hydraulic cylinder rods.
The following table shows changes in the friction coefficient of steel before and after phosphating.
| Performance Indicator | Before Phosphated | Post-Phosphating (Manganese-Based Heavy-Coating) |
| Friction Coefficient | 0.15-0.25 (Steel-on-Steel) | 0.05-0.08 (Run-in Condition) |
| Anti-Galling Capacity | Susceptible to breakdown | Withstands > 50 MPa Contact Stress |
| Wear Amount | 100 mg / 10,000 cycles | < 15 mg / 10,000 cycles |
3.4. Improving Lubrication
The porous structure of the phosphate coating can absorb and retain lubricants like engine oil and grease, enhancing lubrication properties.
It will reduce friction between metal components, preventing excessive wear and lowering the likelihood of mechanical failure.
For example, after manganese phosphating, machine tool guide rails can experience a 2-3 times increase in wear resistance.
Bolts treated with phosphating have reduced friction during assembly, minimizing thread damage.
3.5. Cost-Effective and Environmentally Friendly
Compared to other surface treatments (such as electroplating or spraying), the phosphating process is relatively low-cost in terms of equipment and material requirements.
As shown in the table below, the equipment investment for phosphating is less than 1/10th that of chrome or nickel plating lines.
| Comparison of Surface Treatment Processes | ||
| Comparison Item | Chrome/Nickel Plating | Phosphating Treatment |
| Equipment Investment | > RMB 5 million | < RMB 500,000 |
| Energy Consumption | 20-30 kWh/m² | 1-3 kWh/m² |
| Wastewater Toxicity | Contains Cyanide/Hexavalent Chromium | Can be made Chromium-Free |
| Processing Speed | 1-2 hours/batch | 3-15 minutes/batch |
Modern low-zinc, nickel-free, and nitrite-free phosphating processes can be performed at ambient to moderate temperatures, reducing harmful emissions and meeting environmental standards like the EU RoHS.
3.6. High Material Compatibility
Phosphating is not only suitable for ferrous metals like carbon steel and cast iron but can also be applied to non-ferrous metals such as aluminum and zinc alloys (using specialized phosphating solutions).
This makes it versatile for a variety of industries, including automotive, machinery, home appliances, and hardware.
We will provide a list of materials suitable for phosphating in the coming sections.
3.7. Enhancing Fatigue Resistance
The micro-porous structure of the phosphate coating can absorb vibrational energy.
For components subject to high loads and repeated motion (e.g., automotive chassis and aerospace parts), the phosphate film provides additional surface strength, reducing fatigue and delaying the formation and propagation of cracks, thus improving the part’s resistance to fatigue.
3.8. Good Electrical Insulation
The electrical resistivity of the phosphate coating ranges from 10⁶ to 10⁸ Ω·cm, which provides effective electrical insulation.
In applications where electrical interference or conductivity needs to be minimized, phosphating can reduce current leakage and enhance the insulation performance of components.
A typical example is the use of phosphating in motor silicon steel sheets to prevent short circuits.
3.9. Improved Rust Resistance
Especially in zinc phosphating, the phosphate coating forms a protective coating similar to zinc, providing excellent rust resistance.
For metal components exposed to air and water, phosphating provides long-lasting rust protection, significantly reducing the risk of corrosion.
3.10. Improved Aesthetic Appearance
Phosphating creates a uniform gray, black, or green finish on the metal surface, which not only improves the appearance of the component but also meets specific aesthetic requirements in some applications, such as decorative coatings or visual effects.
Further knowledge on phosphating coating colors will be explored in more detail soon.
4. Classification of Phosphating
4.1. Phosphating Classification Based on Film-Forming Metal Ions
4.1.1. Zinc Phosphate Conversion Coating
Zinc phosphate conversion coating, also called Zinc-based phosphating, with zinc salts (such as zinc phosphate) as the main component, is the most widely used phosphating type. It is particularly suitable for galvanized steel or aluminum alloys.
The zinc phosphate coating is thin and typically appears blue, green, or gray, offering excellent corrosion resistance.
Zinc phosphating can be classified into low-zinc (thin film, suitable for electrophoretic coating), medium-zinc, and high-zinc (thicker film, stronger rust resistance).
Zinc-Based Phosphating Advantages: Good rust resistance, excellent adhesion to coatings, making it ideal for pre-coating layers (such as automotive body panels and home appliance housings).
Zinc phosphating is commonly used in industries like electronics, automotive, and construction to effectively prevent metal rusting.
Zinc-Based Phosphating Film Characteristics: Ranges from light gray to dark gray, typically 1-15μm in thickness, uniform, dense, and with strong adhesion.
4.1.2. Manganese Phosphate Coating
Manganese phosphate coating, also named Manganese-based phosphating uses manganese salts as the primary component, with manganese iron phosphate salts as the primary product.
Manganese phosphating is ideal for applications requiring high wear resistance and lubrication.
Manganese phosphate coatings are generally thicker and more durable than iron phosphate coatings and are commonly used in engine components, automotive chassis, and other applications requiring wear resistance.
Manganese phosphate films are usually gray-black or dark gray, with a thickness range of 2-20μm, high hardness, and excellent wear resistance.
Its lubrication properties make it ideal for parts subject to sliding or friction (such as gears, bearings, bolts) and can also serve as a coating base layer for rust resistance.
4.1.3. Iron Phosphate Conversion Coating
Iron phosphate conversion coating, also called Iron-based phosphating, which uses iron salts as the main component, is commonly applied to steel and iron alloys, forming an iron phosphate coating.
Iron phosphate conversion coating is widely used for corrosion protection in automotive parts, home appliances, and machinery.
Iron phosphate coatings generally appear blue-gray, gray, or black and are thin (0.1-1μm) with a porous structure.
Fe-based Phosphating Advantages: Simple process, low cost, and suitable for short-term rust prevention or as a coating base layer (e.g., steel plate pre-treatment). However, its rust resistance is weaker compared to zinc-based coatings.
4.1.4. Zinc-Calcium Phosphating
A modified version of zinc phosphating, this process uses both zinc salts and calcium salts as the main components.
Zinc-Calcium phosphating coatings are environmentally friendly, reducing harmful substances like nickel and nitrites.
Zinc-Calcium Phosphating Advantages: Ideal for applications with strict environmental requirements, such as pre-treatment for coatings in automotive parts and hardware.
4.1.5. Aluminum-Based Phosphating (Al-based Phosphating):
Aluminum-based phosphating is used for aluminum and aluminum alloys, with the goal of enhancing surface adhesion and corrosion resistance, while also improving hardness. The aluminum phosphate film typically appears light gray.
Which is the most widely used phosphate conversion coating? The answer is Zinc phosphate conversion coating.
Zinc phosphating is the most popular due to its relatively simple process, low cost, and high cost-effectiveness, providing rust protection similar to galvanizing, making it especially suitable for steel and iron alloys.
Manganese phosphating is also widely used for parts requiring high wear resistance, such as automotive components and machinery.
Iron phosphating and aluminum phosphating are less commonly used in practical applications.
4.2. Classification Based on Film Weight
Phosphating can be classified by the weight of the coating into light, medium, and heavy film types.
Characteristics | Light Film Phosphating (Film Weight < 1g/m²) | Medium Film Phosphating (Film Weight 1-10g/m²) | Heavy Film Phosphating (Film Weight > 10g/m²) |
Film Thickness | 0.1-1μm | 1-10μm | 10-30μm |
Film Weight | < 1g/m² | 1-10g/m² | > 10g/m² |
Common Types | Iron-based Phosphating | Low Zinc Phosphating, Medium Zinc Phosphating | Manganese Phosphating |
Corrosion Resistance | Weak, suitable for short-term rust protection | Moderate, suitable for long-term rust protection and coating base layers | Strong, suitable for high-load and friction parts |
Wear Resistance | Poor, mainly used as a base layer or for short-term protection | Moderate, suitable for daily use | Excellent, suitable for wear and lubrication applications |
Coating Adhesion | Good, ideal as a coating base layer | Very good, balancing corrosion protection and adhesion | Poor, usually not used for coatings |
Main Uses | Coating base layer, short-term rust protection | Rust protection, improving coating adhesion | High wear resistance, lubrication, suitable for mechanical parts |
Application Fields | Home appliances, automotive, construction (short-term rust protection) | Automotive parts, home appliances, steel structures | Mechanical parts, high-load moving components, gears |
Film Characteristics | Extremely thin, mainly for coating adhesion | Medium thickness, balancing corrosion and coating effects | Thick film, providing excellent wear and lubrication properties |
Common Substrates | Steel, cast iron, etc. | Steel, aluminum alloys, cast iron | Steel, aluminum alloys, heavy machinery components |
4.3. Classification Based on Treatment Temperature
High-Temperature Phosphating: Treatment temperature 80-90°C, thicker films, strong adhesion, but high energy consumption and complex processes. This method is gradually being replaced.
Medium-Temperature Phosphating: Treatment temperature 50-70°C, balanced performance, widely applied (e.g., some zinc and manganese phosphating).
Low-Temperature Phosphating: Treatment temperature 15-40°C, low energy consumption and high efficiency, the current mainstream direction (e.g., environmentally friendly zinc phosphating).
Room Temperature Phosphating: Performed at room temperature, simple process, but lower coating stability, suitable for low-demand applications.
4.4. Classification Based on Function
Protective Phosphating: Focuses on enhancing metal corrosion resistance, with thicker films (such as manganese-based, high-zinc phosphating). It can serve as a short-term rust protection layer or be combined with oil coatings for enhanced rust prevention (referred to as “oily phosphating”).
Pre-Coating Phosphating: Provides a good base layer for subsequent coatings (such as painting, electrophoretic coating), improving adhesion and preventing peeling or bubbling (e.g., zinc-based, zinc-calcium phosphating).
Functional Phosphating: Imparts special functions to the surface, such as wear resistance and lubrication in manganese-based phosphating, ideal for moving parts (gears, pistons), and friction reduction in iron-based phosphating for cold working lubrication.
5. A List of Materials Suitable for Phosphating
Phosphating is primarily applicable to ferrous metals, where the core process involves generating a phosphate conversion film on the metal surface through chemical reactions. Non-ferrous metals, however, are generally unsuitable for phosphating unless special pre-treatment is applied.
5.1. Carbon Steel
Carbon steel is the most commonly used base material for phosphating, including low-carbon steels (such as 1018 steel, 1020 steel), medium-carbon steels (e.g., S45C), and high-carbon steels.
Phosphating on carbon steel is quick and results in a uniform film layer, making it a primary target for phosphating treatments.
5.2. Cast Iron
Cast iron, like gray cast iron, ductile iron with its high carbon content and porous surface, benefits from phosphating, as the phosphating film fills the pores and enhances both rust resistance and coating adhesion.
Note: The phosphating film on cast iron may appear darker (gray-black to black), and the film thickness is slightly greater than that on carbon steel.
5.3. Alloy Steel
Low-alloy structural steels (such as 45# steel, 20Cr, etc.) can be effectively phosphated. However, high-alloy steels (e.g., stainless steel, high-chromium steel) often have a passive oxide film on their surface that inhibits the phosphating reaction.
In these cases, the passive oxide film must be removed, typically through pickling or acid treatments, to enable effective phosphating.
5.4. High-Alloy Steel
Although high-alloy steel, like stainless steel and tool steel are ferrous metal, stainless steels with chromium content ≥12% form a dense passivation layer (Cr₂O₃) on the surface, which prevents the phosphating solution from reacting with the base metal.
To successfully phosphatize, the passivation film must be removed through processes such as sandblasting, electrolytic activation, or strong acid etching (however, this process is complex and less commonly used in practical applications).
5.5. Non-Ferrous Metals
Non-ferrous metals like aluminum, copper, zinc, and magnesium exhibit extremely weak or no phosphating reaction.
Typically, these metals are treated with anodizing, passivation, or other specialized surface treatment processes instead of phosphating. If phosphating is necessary, special pre-treatment steps must be taken.
Below is a list of materials suitable for phosphating:
| Material Type | Material | Phosphating Suitability | Phosphating Process | Coating Characteristics | Precautions |
| Ferrous Metals | Low Carbon Steel | ★★★★★ | — | — | Best results for 1020/Q235, etc. |
| Cast Iron | ★★★★☆ | — | — | Requires enhanced acid washing to remove graphite. | |
| Alloy Steel | ★★★☆☆ | — | — | High Cr/V content may inhibit phosphating reaction. | |
| Stainless Steel | ★★☆☆☆ | — | — | Requires pre-activation (oxalic acid etching). | |
| Non-Ferrous Metals | Aluminum Alloy | — | Chromate phosphating / Chromium-free zirconium phosphating | Coating thickness 0.3-1μm (golden yellow) | — |
| Zinc Alloy | — | Low-temperature zinc phosphating | Prevents over-corrosion (time < 2min) | — | |
| Magnesium Alloy | — | High manganese conversion coating | Requires specialized fluorine-containing phosphating liquid | — | |
| Copper & Alloys | — | Iron-based phosphating | Coating shows a rainbow color | — | |
| Materials Unable to be Phosphated | Titanium & Titanium Alloys | — | — | — | Due to the chemical properties of titanium, phosphating film cannot be formed. |
| High-Silicon Cast Iron | — | — | — | Si > 14% will inhibit phosphating reaction. | |
| Non-Metallic | — | — | — | Materials like plastics, ceramics, etc., cannot undergo phosphating. |
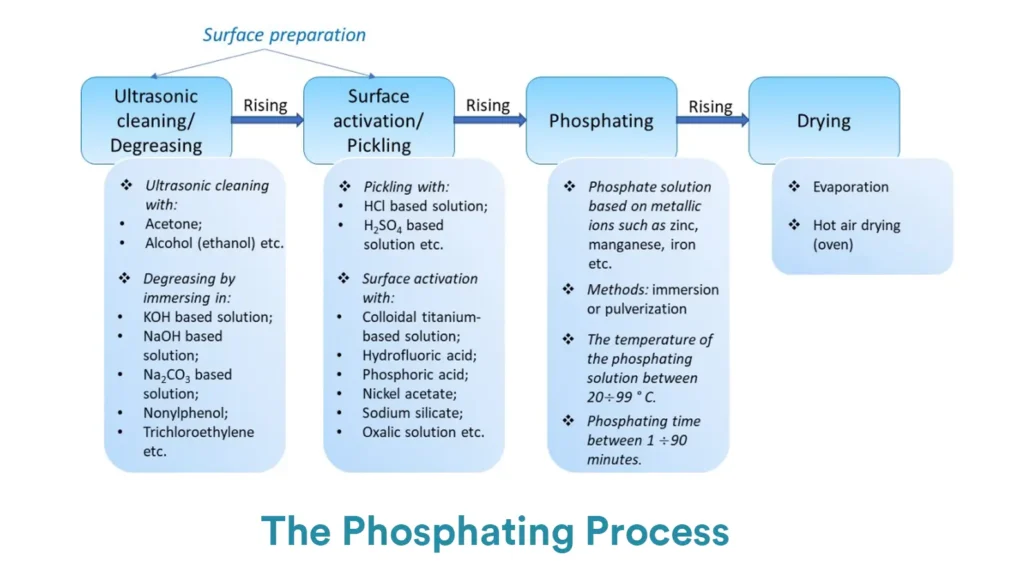
6. Phosphate Conversion Coating Process
The core process of phosphating generally includes three major stages: pre-treatment, main phosphating treatment, and post-treatment.
6.1. Pre-treatment
Phosphating pre-treatment is going to remove oil stains, oxide scale, rust, dust, and other contaminants from the metal surface, ensuring that the phosphating solution can directly contact the metal substrate to form an even, dense phosphating film.
The main steps of phosphating pre-treatment include:
Degreasing (Oil Removal)
The main goal of degreasing is to remove oils, such as animal and plant oils or mineral oils, from the metal surface (as oil contamination can hinder the phosphating reaction and result in an incomplete coating).
Common degreasing methods are listed below.
Alkaline Degreasing: Soaking or spraying with alkaline solutions such as sodium hydroxide or sodium carbonate at room or medium temperatures, removing grease through saponification.
Solvent Degreasing: Using organic solvents (e.g., gasoline, trichloroethylene) to dissolve oils (suitable for precision parts or heavy oil contamination).
Emulsifying Degreasing: Using solutions containing surfactants to emulsify oils (operates at room temperature and is more environmentally friendly).
Water Rinsing
After degreasing, rinse the workpiece with clean water to remove any residual degreaser (to avoid bringing alkaline substances into subsequent acid washing or phosphating solutions, which could affect solution stability).
Typically divided into “primary rinsing” and “secondary rinsing,” the latter helps improve cleanliness.
Acid Pickling
Workpieces with oxide scale or rust require acid pickling to remove oxide films (such as Fe₃O₄ on hot-rolled steel) and iron rust (Fe₂O₃·nH₂O).
Common acids used include hydrochloric acid and sulfuric acid (concentration: 5%-15%), with corrosion inhibitors (such as urotropine) added to prevent excessive corrosion.
Note: Cold-rolled steel or precision parts without rust can skip this step to avoid excessive corrosion affecting dimensional accuracy.
Water Rinsing (after acid pickling)
Rinse off any residual acid and corrosion products (such as iron salts) to prevent contamination of the phosphating bath (acid will lower the pH of the phosphating solution, and iron salts will make the film rough).
6.2. Phosphating
Immerse or spray the pre-treated parts in the phosphating solution, and a chemical reaction will form a phosphating coating on the surface.
Depending on the type of phosphating, the temperature, time, and pH need to be adjusted.
As the reaction progresses, a uniform phosphate film gradually forms on the metal surface (e.g., zinc-based films appear gray, manganese-based films appear dark gray or black).
The quality can be judged by the appearance of the coating (e.g., no exposed base, no spots).
Generally, spraying is faster than immersion, but immersion provides better corrosion resistance and adhesion.
6.3. Post-treatment
Post-treatment will enhance the performance of the coating and extend its lifespan.
Post-treatment includes water rinsing, passivation, and drying.
Water Rinsing
Rinse the workpiece surface to remove any residual phosphating solution, preventing the formation of white haze after drying (which can affect appearance and subsequent treatments).
Passivation
Use chromate or chromium-free passivation solutions (e.g., zirconium salts, silanes) to treat the phosphated film, sealing the film’s pores, further enhancing corrosion resistance, and improving adhesion to coatings.
Drying
Remove moisture from the workpiece surface to prevent the uncoated phosphating film from rusting in humid environments.
Common drying methods include natural air drying or hot air drying (at temperatures of 60-120°C, avoiding high temperatures that could cause cracking of the film).
7. Phosphating Coating Colors
As mentioned earlier, different phosphating films have different colors. Below is a list of phosphating coating colors we compiled after consulting with the engineers at the phosphating plant.
| Phosphating Type | Typical Color | Cause of Color |
| Zinc-based Phosphating | Light Gray → Dark Gray | The natural color of zinc phosphate crystals (Zn₃(PO₄)₂); the thicker the film, the darker the color. |
| Manganese-based Phosphating | Black Gray → Dark Black | High-density crystallization of manganese iron phosphate (Mn₂Fe(PO₄)₂) + oil absorption in the pores. |
| Zinc-Calcium Phosphating | Grayish White → Silver Gray | Calcium ions refine the crystal structure, enhancing reflectivity. |
| Iron-based Phosphating | Rainbow → Blue Purple | Light interference effects of the amorphous iron phosphate film. |
The image below show a batch of CNC machined parts we recently made. The material used is 1020 steel, and the process applied was thick phosphating with manganese-based phosphating.

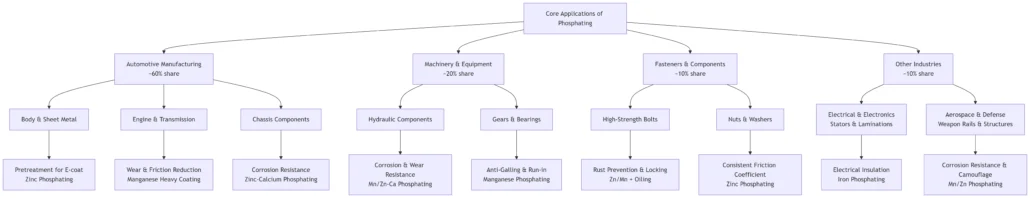
8. Industries that Use Phosphating
Phosphating treatment improves the corrosion resistance, wear resistance, lubricity, and adhesion of metal materials, making it crucial in industries such as automotive, machinery manufacturing, and fasteners.
8.1. Automotive Industry
The automotive manufacturing industry is the largest user of phosphating, accounting for approximately 60% of the total applications.
Below are three typical applications of phosphating in the automotive industry:
8.1.1. Pre-treatment for Body Coating
Pre-treatment for body coating is the most classic and largest-scale application of phosphating.
It involves applying low-temperature zinc-based phosphating to stamped steel parts of the vehicle, such as doors, hoods, and longitudinal beams, to form a thin film of about 2-3μm.
First, this microcrystalline conversion coating provides an excellent adhesion base for subsequent electrophoretic coating, preventing paint film detachment through mechanical anchoring.
Second, it significantly enhances the overall corrosion resistance of the vehicle body by isolating the metal substrate from the external environment and preventing rust from spreading from the inside.
8.1.2. Engine Components
Crankshafts, camshafts, piston rings, gear synchronizer rings, and other engine components are treated with medium-to-high-temperature manganese-based thick-film phosphating and immersed in lubricating oil.
This treatment reduces friction, improves wear resistance, prevents dry friction and “seizing” during initial operation, and ensures short-term normal operation even when lubrication is insufficient, thanks to the porous structure that stores oil.
This process effectively reduces the friction coefficient by 60%, doubling the service life of the parts and ensuring the durability and stability of engine components.
8.1.3. Chassis Parts
Chassis parts such as screws, bolts, nuts, springs, and brake system components are treated with zinc-calcium or manganese-based phosphating, followed by black sealing treatment and rust-proof oil coating.
This approach effectively resists stone impact, mud, and snowmelt salt corrosion. Not only does it enhance the corrosion resistance of the parts, but it also ensures their long-term use in harsh environments, passing over 480 hours of salt spray testing to guarantee excellent protective performance.
8.2. Machinery and Equipment Manufacturing
8.2.1. Hydraulic System Components
Hydraulic components such as hydraulic cylinder piston rods and valve cores are treated with manganese-based phosphating to improve wear resistance and resist friction from dust seals, ensuring long-term operation in high-friction environments.
Additionally, phosphating enhances rust resistance, ensuring hydraulic components can resist rust even when stored and operated in humid environments. Manganese-based phosphating is widely used as a low-cost alternative to chrome plating in the construction machinery sector, such as excavators and cranes.
8.2.2. Gears and Bearings
Heavy-duty gears and sliding bearings are treated with manganese-based thick-film phosphating to prevent “cold welding” and galling under high-contact stress.
The phosphated film provides excellent running-in performance, effectively reducing friction, improving wear resistance, and extending the service life of components. This treatment is widely used in gears and bearings in high-load machinery.
8.3. Fastener Industry
In the fastener industry, grade 10.9 and 12.9 hexagon bolts undergo zinc-based or manganese-based phosphating treatment, followed by a coating of MoS₂ (molybdenum disulfide) grease to improve rust resistance and ensure long-term stability in harsh environments.
The phosphating film controls the friction coefficient of the bolts, ensuring accurate pre-tightening force and preventing loosening.
Compared to galvanizing, the phosphating film performs better in micro-motion wear resistance and is widely used in industrial applications requiring precise fastening, complying with DIN 267 Part 10 and ISO 4042 standards.
8.4. Electrical Industry
In the electrical industry, motor stator and rotor cores (silicon steel sheets) are treated with iron-based phosphating or insulation coatings to form an insulating layer between the silicon steel sheets, reducing eddy current losses and improving motor efficiency.
Phosphating treatment effectively improves motor efficiency and reduces energy consumption, widely applied in motors and transformers.
8.5. Military and Aerospace
In the military and aerospace sectors, parts such as weapon rails, internal structural components, and springs undergo manganese-based phosphating (black, wear-resistant and friction-reducing) or zinc-based phosphating (rust-resistant) treatment.
The primary purposes are rust prevention, wear resistance, and dulling. The phosphating coating enhances the reliability of equipment during long-term storage, reduces friction and wear of moving parts, and the black phosphating coating also reduces light reflection for concealment.
9. Considerations for Phosphating CNC Parts
Phosphating treatment improves the corrosion resistance, wear resistance, lubricity, and adhesion of metal materials, making it crucial in industries such as automotive, machinery manufacturing, and fasteners.
As discussed earlier, phosphating comes in different types. Therefore, when a customer requests phosphating for custom CNC parts, we need to pay attention to the following points:
To the Customer:
The customer needs to confirm the type of phosphating required, whether it is manganese-based, zinc-based, or iron-based phosphating. The thickness of the phosphating film also needs to be confirmed with the customer.
Allowance for Tolerances:
Phosphating may increase the dimensions of the parts, so it is important to consider the phosphating coating thickness during CNC machining and make the necessary allowances, so the required CNC machining tolerance can be met.
Phosphating Plant:
To ensure the quality of the phosphating and maintain the additional tolerances required by the CNC machining, the phosphating plant can be asked to conduct small batch phosphating first. Once the test batch is approved, larger-scale phosphating can proceed.
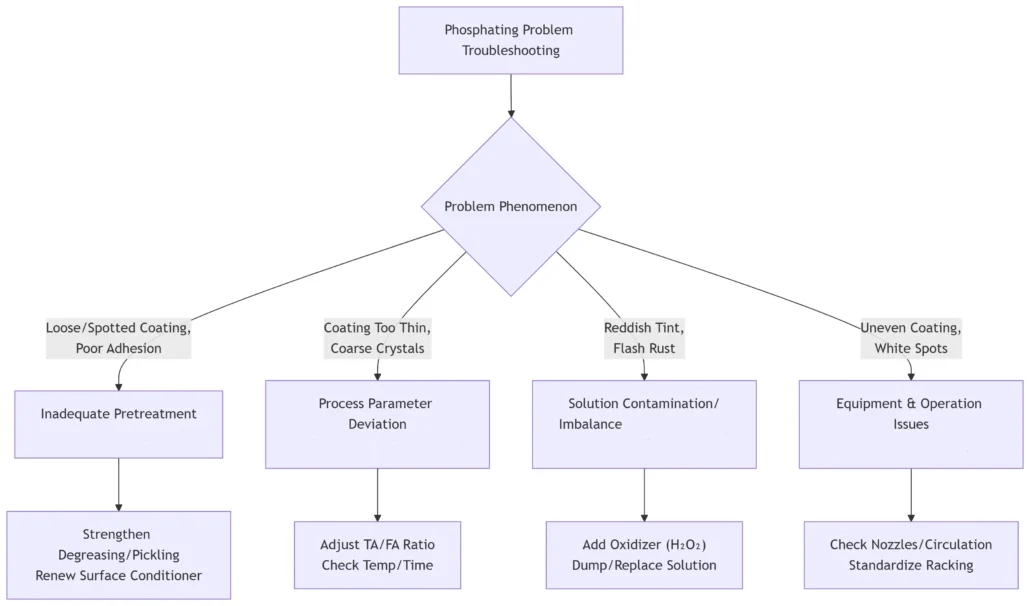
10. Common Phosphating Issues
Common issues in phosphating include loose coating, mottling, poor adhesion, coating that is too thin, coarse crystallization, red discoloration, rust return, uneven coating, white spots, etc. The following is an analysis of common causes.
After communicating with engineers from multiple phosphating plants, we have summarized a quick reference chart for common phosphating problems, their causes, and solutions.
| Issue Category | Typical Phenomenon | Root Cause | Solution |
| Poor Pre-treatment | Mottling, uneven coating, white spots | Incomplete degreasing, residual oil on the surface | Strengthen degreasing (increase temperature and concentration); clean floating oil from the degreasing tank; ensure proper spraying pressure |
| Coarse crystallization, poor adhesion | Inadequate rust removal or ineffective surface conditioner (insufficient Ti⁺, pH imbalance) | Strengthen acid pickling control; immediately replace or replenish surface conditioner; ensure clean water rinsing after surface conditioning | |
| Out-of-Control Process Parameters | Too thin coating | Low total acidity (TA) or high free acidity (FA); insufficient temperature/time | Add phosphating agents to adjust TA; neutralize FA with Na₂CO₃; strictly monitor temperature and time |
| Too thick coating, gray residue | Low FA; high TA; excessive temperature/time | Add phosphoric acid to increase FA; adjust process parameters to standard range | |
| Excessive sediment in the bath | Low FA; high Fe²⁺ ion concentration | Add acid to adjust acid ratio; add hydrogen peroxide (H₂O₂) to oxidize and remove debris; regularly empty the bath | |
| Coating Appearance and Performance Defects | Red or yellow surface | Cu²⁺ ion contamination (equipment corrosion or handling copper-containing parts); high Fe²⁺ | Inspect and replace copper components in equipment; electrochemical treatment or chemical precipitation of Cu²⁺; use H₂O₂ to oxidize Fe²⁺ |
| Poor corrosion resistance, early rusting | Thin/uneven coating; poor post-treatment (ineffective passivation or lack of oil immersion) | Solve film formation issues; inspect and adjust passivation solution; ensure sufficient rust-proof oil immersion at appropriate temperature (40-60°C) | |
| White residues (after-rust) | Incomplete water rinsing after phosphating, leaving soluble salts on the surface | Use deionized water for final rinsing; increase water rinsing temperature and pressure; add hot water drying process |
To achieve the best phosphating results, engineers have advised us to always remember:
“70% Pre-treatment”: The majority of issues stem from incomplete degreasing, rust removal, and surface conditioning.
“Process is Key”: Closely monitor the three core parameters: TA/FA ratio, temperature, and time.
“Post-treatment is Crucial”: Proper passivation and oil coating after phosphating are the final safeguards for performance.
11. Phosphating Technology Trend
The future of phosphating technology will follow four main trends:
Environmental Protection
Intelligent Control
Low Temperature Processing
Resource Efficiency
Phosphating will advance through phosphorus-free alternatives and breakthroughs in nanotechnology to overcome performance limitations.
12. Phosphate Conversion Coating Service for CNC Machining Parts
In this blog, we have explored the various aspects of Phosphate Conversion Coating, including its types, applications, and the benefits it brings to CNC machined parts.
If you’re looking for a reliable partner for precise CNC parts with phosphating treatment, contact us today. Let us help you enhance the performance of your CNC machined parts with our high-quality phosphate conversion coating service!

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He also spent three years in CNC engineering, quoting, design, and project management. Lucas holds an associate degree in mold design and has self-taught knowledge in materials science. He’s a lifelong learner who loves sharing his expertise.



