Table of Contents

In metal machining, surface finishing techniques enhance the appearance and performance of metals. Among these crucial methods, electropolishing plays a significant role in both high-end precision CNC-machined components and everyday products.
Electropolishing can significantly improve the appearance of parts and certainly enhance the corrosion and abrasion resistance.
This article will introduce everything you should know about electropolishing.
Key Takeaways
Electropolishing is an electrochemical process that removes microscopic surface peaks from metals, resulting in a mirror-like smooth finish, improved corrosion resistance, and reduced contamination risk.
Typical material removal depth ranges from 5–50 μm, achieving up to 40–50% improvement in surface roughness (Ra) depending on the material and process parameters.
Electropolishing serves as both a finishing and passivation method, especially for stainless steels, and is standardized under ASTM B912 and ASTM A967.
Compared to mechanical polishing, electropolishing provides superior consistency, can handle complex geometries, and leaves a clean, passive surface ideal for medical, semiconductor, and food-grade components.
1. What is Electropolishing?
Electropolishing is a type of electrochemical finishing technique that can remove the majority of imperfections from metal parts, making the surface shiny, smooth, and clean.
Electropolishing, also known as electrochemical polishing, electro polishing, anodic polishing, or electrolytic polishing, is a process that mainly depends on the application of direct current within an electrolyte solution.
During the electropolishing process, the metal workpiece is immersed in a special electrolyte solution and acts as the anode (positive electrode), while an insoluble metal, such as lead or stainless steel, serves as the cathode (negative electrode).
When direct current flows through the electrolyte, the metal at the anode would dissolve selectively. Because microscopic peaks on the metal surface experience higher current density, they dissolve faster than the valleys.
Over time, the microscopic roughness would be preferentially shaved due to such an uneven dissolution. Finally, the surface would be smoother and brighter.
In simple terms, electropolishing is a microscopic sculpting process which uses electric current and electrolyte together to gently remove surface imperfections and irregularities.
Electropolishing is particularly useful for polishing and deburring components that are too fragile or complex to be mechanical polished.
It can be seen as reverse electroplating that uses electric current to dissolve a thin layer of metal ions into an electrolyte solution, but not to form a thin coating on the surface.
Depending on the processing parameters, electropolishing can at most remove up to 40 micrometers coating from the surface. But 8 to 20 micrometers are the typical range.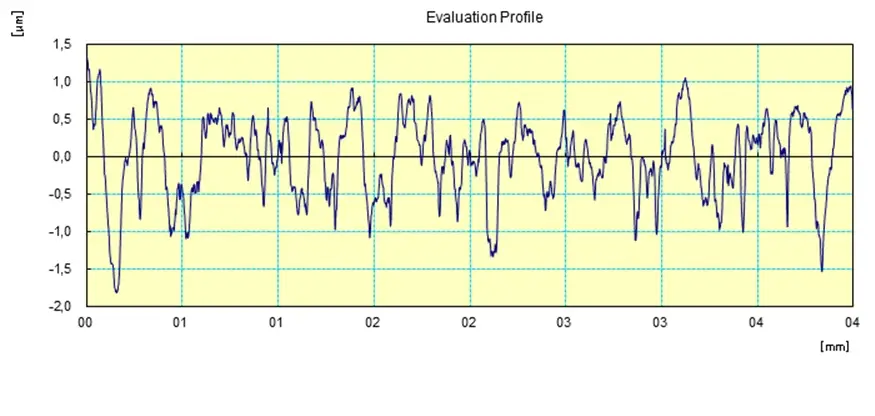
The level of finish polishing depends on the initial finish. The percentage of surface finish can usually achieve between 25 to 40% Ra(Roughness Average) reduction. The highest level is 50%.
However, as the Ra value becomes smaller, the polishing effect would be worse with diminishing rate of return. In other words, an already well-finished part will not benefit as much as a relatively rougher part from electropolishing.
Therefore, electropolishing is commonly used as a secondary finishing process for mechanical polishing. Mechanical polishing is applied as preliminary finishing process to remove macroscopic imperfections, while electropolishing is used to remove microscopic imperfections.
2. How Does Electropolishing Work?
Electropolishing uses an electrochemical principle to smooth and brighten the metal surface. By applying direct current to a specific electrolyte solution, selective dissolution occurs on the metal part that acts as the anode.
The process preferentially removes microscopic surface peaks, resulting in a smooth, bright, and clean metal surface.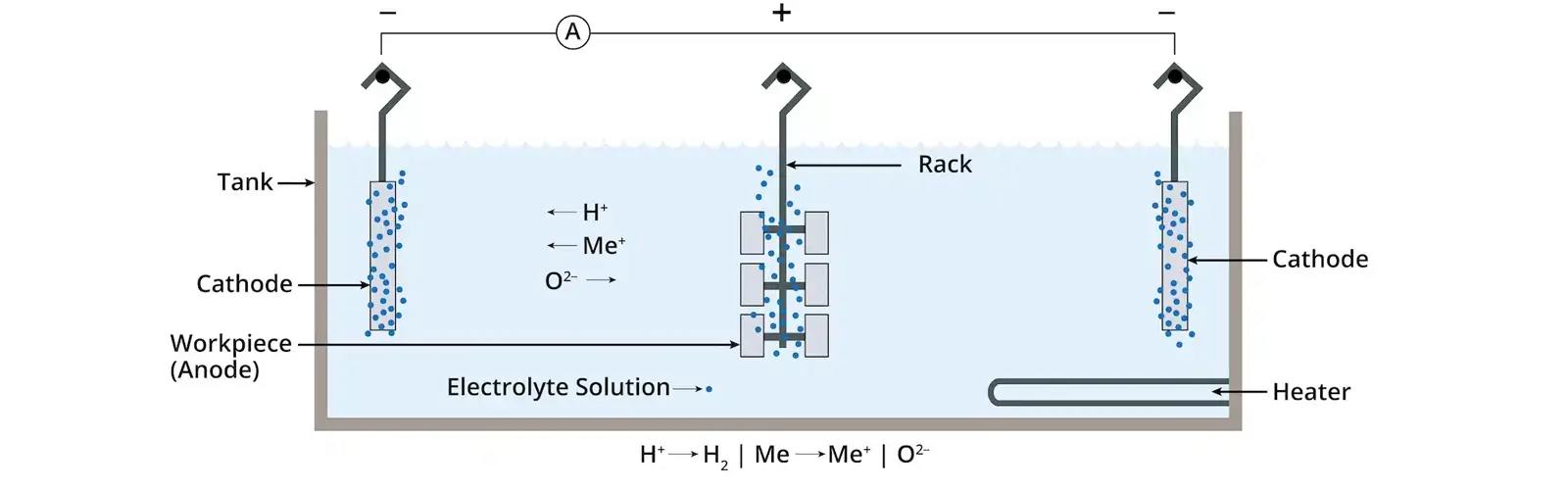
2.1. What is the Working Principle of Electropolishing?
Within the electropolishing process, the metal part serves as the anode, connected to the positive terminal of a DC power rectifier.
While an item made of stainless steel or zirconium would act as the cathode connected with the negative terminal. Then both the anode and cathode are immersed in an electrolyte bath whose temperature is strictly controlled.
The electropolishing chemicals for stainless steel are usually composed of a high-viscosity mixture of sulfuric acid and phosphoric acid.
Once as the direct current is applied, a series of complex chemical reactions would occur on the anode surface.
Taking iron (Fe) as an example. Oxidation would take place at the anode where the metal loses electrons and ions leave the metal lattice and then enter the electrolyte.
As the reaction proceeds, anions in the electrolyte combine with the dissolved metal ions to form various metal salts. These salts gradually accumulate on the anode surface and form a viscous film with certain thickness and consistency.
This viscous film plays a crucial role in the electropolishing process. Its thickness is not uniform. It is thinner on microscopic surface peaks and thicker in valleys.
Since the film has relatively high electrical resistance, it affects the distribution of current density across the metal surface. On the peaks, where the film is thin, the resistance is low, leading to higher current density and a faster rate of metal dissolution.
Conversely, in the valleys, the film is thicker, with higher resistance, so the metal ions would dissolve more slowly. This selective dissolution gradually smooths the microscopic roughness of the surface, making it polished.
2.2. What is the Process of Electropolishing?
2.2.1. Surface Preparation
Degreasing: Remove oils, greases, cutting fluids, and other contaminants from the workpiece surface using degreasers, alkaline cleaners, or solvents.
Rinsing: Thoroughly rinse the workpiece with clean water to remove any remaining cleaning agents.
Acid Pickling(Optional): For workpieces with oxide scales, weld discoloration, or heavy rust, acid pickling or descaling may be required to restore a clean metal surface.
Rinsing Again: Wash off any residues left from the acid pickling process.
2.2.2. Part Fixturing
Fix the workpiece onto a conductive fixture and ensure a good electrical connection with the anode (positive terminal) of the DC power supply. Note to allow it to be fully immersed in the electrolyte. The fixture design is crucial for achieving uniform polishing.
2.2.3. Electropolishing
Immersion in Electrolyte: Immerse the fixed workpiece (anode) and an insoluble metal (cathode), usually lead or stainless stee in a specific The electrolyte is typically a high-viscosity acidic solution, such as a mixture of sulfuric and phosphoric acids.
Application of DC Power: Apply adirect current power supply with appropriate voltage and current density between the two electrodes.
Anodic Dissolution and Polishing: As current flows through the electrolyte, metal ions on the surface of the workpiece would oxidize and dissolve into the electrolyte. During the polishing process, a viscous anodic film will form on the surface.
Due to the concentration of the electric field, the current density on microscopic protrusions is higher than that in recessed areas.
The metal at the protrusions would dissolve faster and be removed preferentially, thus leading to a progressively smoother and flatter surface.
This process allows for the removal of material at the microscopic level to achieve surface leveling, gloss enhancement, and deburring.
2.2.4. Post-process
Initial Rinsing: After removing the workpiece from the electrolyte bath, a quick rinse is usually performed to recover a portion of the electrolyte solution.
Multi-stage Rinsing and Neutralization: Use a large volume of running water (typically counterflow rinsing) or deionized water to thoroughly remove all acidic chemical residues.
An alkaline neutralization may be included to neutralize any remaining acid.
Passivation (Optional): It is especially for stainless steel. Electrolytic polishing process itself can providecertain passivation, but an additional chemical passivation may be performed to form a more stable and thicker oxide film rich with chromium on the metal surface, further enhancing corrosion resistance.
Drying: Finally, dry the workpiece by hot air.
2.2.5. Inspection
Inspect the workpiece surface on gloss, roughness, and deburring quality to ensure it meets the specified requirements.
3. What should be Noticed during the Electropolishing Process?
Electrolyte Density:
Different electrolytes have specific density ranges. For common electrolytic polishing solutions of stainless steel, the typical density falls between 1.68 and 1.72.
Variations in electrolyte density reflect changes in the relative content of solutes and solvents, which in turn affect the conductivity and ion concentration of the solution.
If the density is too low, it usually indicates excessive water content and insufficient solute concentration, which would lead to slower polishing rates and poor surface finish.
Conversely, if the density is too high, the electrolyte becomes more viscous, hindering ion diffusion and compromising polishing uniformity and efficiency.
Electrolyte Condition:
A high-quality electrolyte typically appears slightly yellowand transparent, indicating that all components are evenly dispersed without impurities or precipitation.
If the electrolyte becomes cloudy, discolored, or shows sedimentation, it suggests that its composition has changed. These changes can significantly impair the electrolyte’s performance, resulting in poor polishing quality and even potential corrosion of the workpiece.
PH Value:
The electrolytic polishing solutions are generally acidic. An appropriate pH level ensures proper anodic metal dissolution and the formation of a stable, viscous film.
When the pH is too high, the weak acidity would slow metal dissolution and polishing efficiency. Conversely, when the pH is too low, excessive acidity can cause over corrosion, leading to surface defects such as pitting or roughness.
Current Density:
Different materials require different current densities during electrolytic polishing. Current density directly affects the anodic dissolution rate and surface quality.
When the current density is too low, the metal would remainactive. Then chemical dissolution would dominate over electrochemical dissolution and lead to poor surface brightness.
Conversely, when the current density is too high, intense oxygen evolution occurs, leading to overheating and over corrosion. This not only increases power consumption but may also cause surface defects such as pits or porosity.
Temperature:
The operating temperature of the electrolyte is typically between 50°C and 75°C and should not exceed 90°C. Temperature has a significant impact on the electrolyte’s viscosity, ion diffusion rate, and anodic polarization.
When the temperature is too low, the electrolyte becomes excessively viscous, which hinders the diffusion of anodic dissolution and reduces the polishing efficiency.
Conversely, when the temperature is too high, vapor produced in the tank can push the electrolyte away from the metal surface, and then the dissolution products would diffuse too rapidly, easily leading to pitting corrosion.
Electropolishing Time:
The duration of electropolishing directly affects the polishing quality and efficiency. Typically, the electropolishing time ranges from 5 to 10 minutes.
If the duration is too short, the microscopic peaks on the metal surface cannot be fully dissolved, resulting in incomplete polishing and insufficient smoothness or brightness.
Conversely, if the polishing time is too long, it might decease the production efficiency and cause over corrosion, leading to dimensional inaccuracies and unnecessary surface loss.
The electropolishing time should be adjusted according to the material, shape, size, and surface finish requirements.
Drying the Part Before Immersion:
Before placing the part into the electropolishing bath, it is essential to ensure that any moisture on its surface is completely dried.
Moisture carried by the part can dilute the solution, reduce its density, and alter its chemical composition and performance, ultimately affecting the quality of the electropolishing process.
Area Ratio of Cathode to Anode:
The area ratio between the cathode and anode should be maintained within an appropriate range, typically at 1:2 to 1:3.5. This ratio directly affects the distribution of current across the electrode surfaces.
If the cathode area is too small, the current density on the anode becomes excessively high, potentially causing localized current concentration and overcorrosion on the anode surface.
Conversely, if the cathode area is too large, the anode current density decreases, slowing the polishing rate.
Electrode Spacing:
The distance between the anode and cathode is typically maintained between 10 and 30 centimeters. The electrode spacing affects both the electric field distribution and the resistance of the electrolyte.
Excessively short spacingwould lead to decreased electrolyte resistance and the higher current density, which would cause localized overheating and over corrosion on the anode surface.
Conversely, if the spacing is too wide, the electrolyte resistance would increase. This would lead to higher energy consumption and uneven electric field distribution, thereby affecting the uniformity of the polishing process.
Power Disconnection During Loading and Unloading:
The power supply must be disconnected when loading or unloading workpieces from the electrolytic polishing tank. This is because attaching or removing fixtures while the system is energized can generate electric sparks.
References:
ASTM B912 — Standard Specification for Passivation of Stainless Steels Using Electropolishing. ASTM International.
ASTM A967 — Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts. Galvanizeit.com.
Google — AI Features and Your Website (developer guidelines for AI Overviews). Google for Developers.
Google — Find information in faster & easier ways with AI Overviews (user support page). Support.Google.com.
4. What are the Types of Electropolishing?
This part will introduce the various types of electropolishing on polishing material and electrolyte, as well as two special electropolishing.
4.1. Types of Electropolishing on Materials
4.1.1. Electropolishing of Stainless Steel
Electropolishing of stainless steel is the most common and widely used application of electropolishing. All of 200, 300, and 400 series of stainless steels can be electropolished.
The electrolyte for stainless steel electropolishing is mainly composed of phosphoric acid (H₃PO₄) and sulfuric acid (H₂SO₄).
Phosphoric acid, as the main component, can provide high surface brightness, while sulfuric acid, instead, can improve conductivity but may slightly reduce surface smoothness.
Electropolishing stainless steel enhances surface smoothness and corrosion resistance. It can meet the high cleanliness requirements of industries such as medical, food, and semiconductor manufacturing.
4.1.2. Electropolishing of Aluminum and Its Alloys
Electropolishing of aluminum and its alloys are commonly used for decorative components, optical reflectors, and electronic housings.
It typically employs phosphoric acid systems, acid solution mixtures comprising phosphoric acid and sulfuric acid, or chromic acid solutions , as its electrolyte.
Among these electrolytes, the chromic one is being phased out due to environmental concerns.
During the electropolishing process of aluminum and its alloys, problems such as over corrosion or matte finishing might occur. It requires strict control of temperature and voltage
4.1.3. Electropolishing of Copper and Its Alloys
Electropolishing of copper and its alloys are commonly used for electronic lead frames, decorative components, and heat exchangers.
Their electrolytes are mainly phosphoric acid, chromic acid, or mixed solutions containing glycerol or ethylene glycol.
Note that copper would oxidize easily, so the parts must be rinsed promptly after polishing to prevent discoloration.
4.1.4. Electropolishing of Titanium and Its Alloys
Electropolishing of titanium and its alloys are commonly used for biomedical implants and aerospace applications. The electrolytes are usually systems containing perchloric acid with methanol or ethylene glycol with sulfuric acid. Compared with other electropolishing process, that of titanium is complex and requires an inert atmosphere for protection.
4.2. Types of Electropolishing on Electrolyte
4.2.1. Aqueous Electrolyte Polishing
Aqueous electrolyte polishing uses water as the main solvent, combined with acids such as phosphoric acid, sulfuric acid, or nitric acid and certain additives.
Under the influence of direct current, the metal surface undergoes anodic dissolution, resulting in a smooth and bright finish.
Electrolyte Compositions
Phosphoric acid (H₃PO₄) + sulfuric acid (H₂SO₄), commonly used for stainless steel and aluminum
Nitric acid + phosphoric acid, used for copper alloys
Advantages
- Low cost and readily available raw materials
- Good conductivity and high current efficiency
- Mature process, widely applied to polishing stainless steel, aluminum, and copper
Disadvantages
- Often containing strong acids or even hexavalent chromium, posing issues of corrosion and environmental pollution
- Not suitable for highly reactive metals such as titanium and magnesium
- Might causing hydrogen embrittlement or oxidation issues
Applications
Widely used for stainless steel products requiring high surface smoothness, such as medical instruments, food machinery, and decorative components.
4.2.2. Organic Electrolyte Polishing
Organic electrolyte polishing uses organic solvents such as ethylene glycol, glycerol, formamide, or dimethyl sulfoxide instead of water as the base medium. Conductive salts such as lithium perchlorate or tetraethylammonium salts and small amounts of acid are added to enable anodic dissolution and achieve metal surface polishing.
Electrolyte Compositions
Ethylene glycol + perchloric acid (HClO₄)
Glycerol + phosphoric acid / sulfuric acid
Formamide + chloride salts
Advantages
- No hydrogen embrittlement and oxidation problems
- Suitable for metals sensitiveto water such as titanium, zirconium, tantalum, and magnesium alloys
- Can achieve more uniform surface with less roughness
- Can achieve high precisefinishing for applications such as microelectronics and MEMS
Disadvantages
- Lower conductivity compared to aqueous systems, requiring higher voltages
- Complex waste treatment and recovery processfor flammable and toxic solvents
- Higher cost
Applications
Widely used for aerospace, biomedical implants, microelectronic components, and precision parts made from highly reactive metals.
4.2.3. Chromium-Free Electrolyte Polishing
Chromium-free electrolyte polishing refers to an environmentally friendly electrolytic polishing process that does not contain hexavalent chromium (Cr⁶⁺).
Traditional electrolytic polishing often used electrolytes with chromium. However, hexavalent chromium has the strong carcinogenicity and environmental hazards.
Electrolyte Compositions
- Phosphoric acid+ sulfuric acid + glycerol
- Phosphoric acid+ citric acid
- Ionic liquid
Advantages
- Environmentally friendly, compliant with RoHS and REACH regulations
- Easier waste treatment and disposal process
- Lower health risks
Disadvantages
- Lower polishing efficiency and brightnessthan that of chromium electrolytes, especially for complex parts
- More complex formula for electrolytes
- Potentially higher cost particularly when ionic liquids are used
Applications
Widely used in stainless steel products for export, medical instruments, components contacting with food, and automotive parts where high environmental standards are required.
Comparison Table for Types of Electropolishing on Electrolyte
| Feature | Aqueous Electrolyte Polishing | Organic Electrolyte Polishing | Chromium-Free Electrolyte Polishing |
|---|---|---|---|
| Solvent | Water | Organic solvents | Water or mixed solvents |
| Suitable Metals | Stainless steel, aluminum, copper, etc. | Titanium, magnesium, tantalum, and other reactive metals | Primarily stainless steel |
| Environmental Friendliness | Poor (if chromium-containing) | Moderate | Good |
| Cost | Low | High | Moderate |
| Surface Finish Quality | Good | Excellent | Good to excellent |
| Safety | Corrosive | Flammable and toxic | Relatively safe |
4.3. Two Special Electropolishing
4.3.1. Flash Electropolishing
Flash electropolishing is a type of special electropolishing, characterized by its extremely short treatment duration.
Electropolishing Features
- Flash Duration: Compared to conventional electropolishing, which typically lasts several minutes, flash electropolishing is performed within just a few seconds, or even less than one second.
- Minimal Material Removal: Due to the extremely short processing time, only an ultra-thin layer of material is removed from the metal surface.
- Specific Purpose: The goal is not to achieve a mirror-like finish or significant roughness reduction, but rather to rapidly remove extremely fine surface defects or the altered surface layer with high precision.
- Power Supply: The process needs instantaneousoutput of high current and precise control on polishing time, often using pulsed or time-switched current modes.
- Temperature Control: Due to significant instantaneous heat generation, efficient cooling or the use of low-temperature electrolytes is required.
Advantages
- Extremely short processing time, ideal for mass or inline production.
- Can effectively remove submicron burrs.
Disadvantages
- Not suitable for applications demanding deep leveling or mirror finishes.
- Narrow process window, requiring precise control of time and current.
Specific Applications
- Scale/Heat Tint Removal: For metals such as stainless steel, welding or heat treatment often produces an unsightly brown or blue oxide layer (heat tint) on the surface. Flash electropolishing can rapidly and uniformly remove this oxide layer, restore the metallic luster, and simultaneously achieve surface passivation.
- FIB Removal: In sample preparation for transmission electron microscopy (TEM), thefocused ion beam (FIB) technique often leaves a thin layer of lattice damage, such as dislocations or amorphous regions on the specimen surface. Flash electropolishing is used to precisely strip away this nanometer-scale damaged layer, producing artifact-free and high-quality TEM foils for accurate microstructural analysis.
- Deburring for Precision Components: For miniature parts with extremely tight tolerances or complex geometries, flash electropolishing can rapidly remove micro-burrs and achieve surface passivation without significantly altering the part’s dimensions.
4.3.2. Dry Electropolishing
Dry Electropolishing is a disruptive metal surface finishing technology developed in recent years. The key difference between this process and conventional electropolishing lies in the fact that no liquid electrolyte is used.
Electropolishing Process
- Medium: The parts are placed inside a drum or container filled with dry, porousand sponge-like solid electrolyte polymer particles.
- Ion Exchange: Connect the part to the positive terminal (anode) of a DC power source, while the container or internal electrode serves as the negative terminal (cathode).
- Material Removal: Under the combined action of electric current and mechanical movement, the surface of the part comes into contact with the solid particles, triggering an ion exchange reaction. This electrochemical process selectively removes only the microscopic surface peaks of the metal.
Advantages
- Geometry Preservation: It offers extremely high selectivity, removing only the surface roughness peaks. As a result, the original geometry, edges, and sharp corners of the part are well preserved, unlike conventional liquid electropolishing, which tends to round off sharp features.
- Environmental Friendliness: Dry electropolishing eliminates the challenges and high costs associated with handling acidic waste liquids, making the process far more environmentally friendly.
- Good Uniformity: Dry electropolishing can achieve more uniform and consistent polishing on complex geometries. Because the electric current flows randomly through the solid electrolyte particle, the differences in current density between surface features are reduced, resulting in a more uniform polishingfinish.
- Excellent Finish: Dry electropolishing can reduce the surface roughness to an extremely low level and achieve a mirror-like finish.
Disadvantages
- Minimal Material Removal: Dry electropolishing is primarily used to remove material from surface roughness peaks for micro-level smoothing. It is not suitable for applications requiring substantial material removal.
- Complex Fixtures: Dry electropolishing may require complex fixturing to hold the parts securely, ensuring proper electrical contact and uniform polishing across all surfaces.
Applications
It is suitable for a wide range of metals and alloys, such as stainless steel, titanium, cobalt-chromium alloys, and nickel alloys, and is widely used in medical devices, aerospace, jewelry, and additive manufacturing applications.
5. What are the Pros and Cons of Electropolishing?
5.1. The Key Advantages of Electropolishing
- Enhancing Surface Smoothness:
By reducing the surface roughness by at most 50%, electropolishing can significantly enhance the brightness and smoothness of metal surfacesand achieve a mirror-like finish.
- Removing Burrs and Contaminants:
The process can eliminate tiny burrs, oxide scales, and embedded particles left after machining, enhancing overall surface cleanliness.
- No Mechanical Stress:
As it is an electrochemical dissolution process, it does not cause plastic deformation, tensile stress, or introduce abrasive residues on the like mechanical polishing does, thus preserving the original metallographic structure of the material.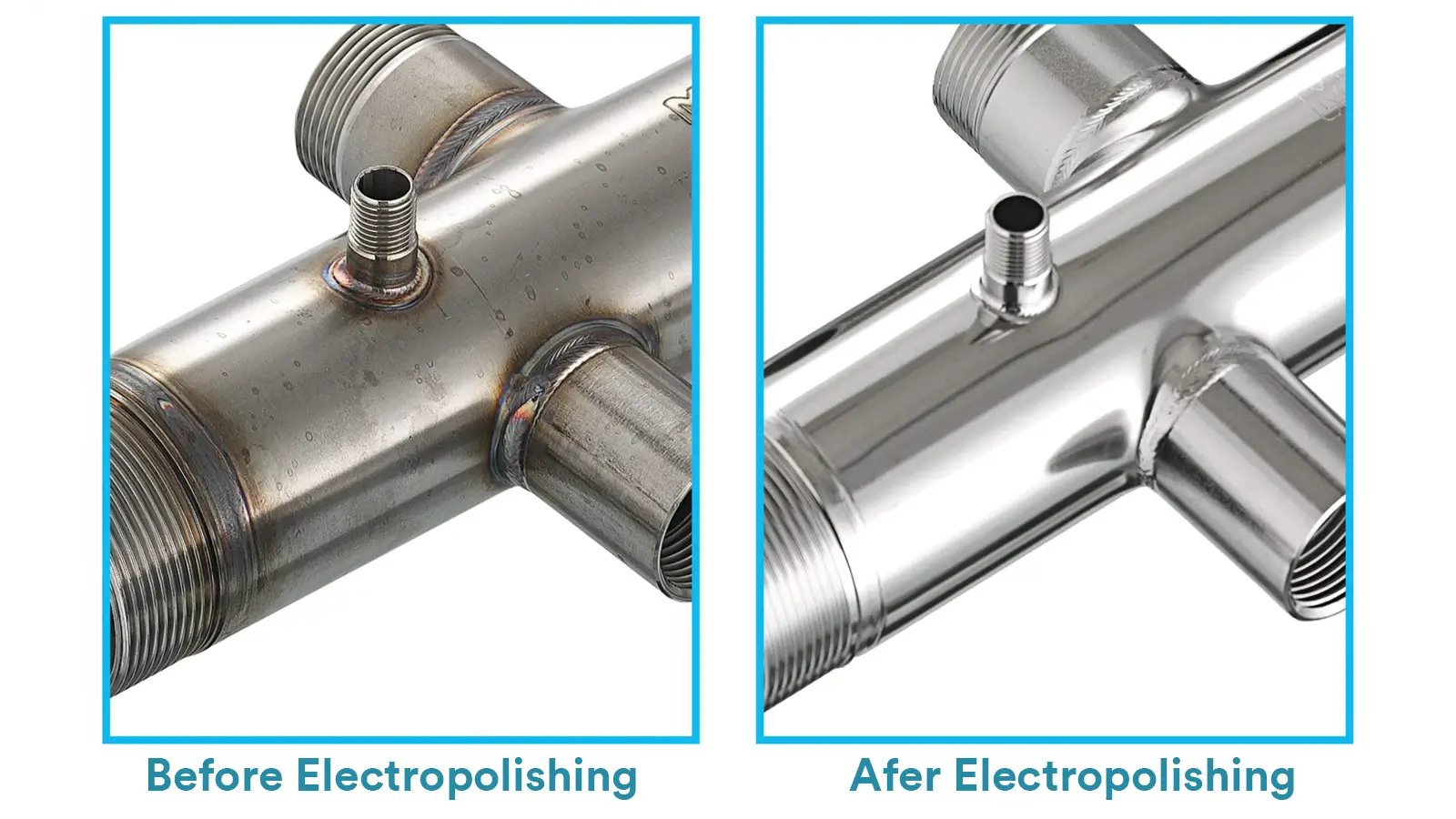
- Good Cleanliness:
Since no abrasives are used, it prevents particle embedding or crosscontamination. Electropolishing is particularly suitable for industries such as food processing, pharmaceuticals, and semiconductors.
5.2. The Key Disadvantages of Electropolishing
- Limitation on Material:
It is mainly suitable for metals capable of passivation, such as stainless steel, aluminum, copper, nickel, and their alloys. But it is less effective or unsuitable for metals that cannot passivate, like carbon steel.
- Unable to Repair Huge Defects:
It cannot fix large surface imperfections such as scratches or pits. Mechanical polishing is usually required beforehand.
- Relatively High Cost:
The process requires specialized electrolytes, power supplies, and equipment. The initial investment and operating costs may be higher than those of simple mechanical polishing.
- Environmental and Safety Concerns:
The electrolytes for electropolishing often contain strong acids that are corrosive or toxic. Waste solutions must be carefully treated, and proper protective measures are required during operation.
6. What are the Applications of Electropolishing?
Medical Instruments and Implants:
Electropolished parts are suitable for medical instruments and implants that have extremely high requirements for surface smoothness and biocompatibility such as surgical tools, orthopedic implants, dental instruments, and catheters.
Food and Beverage Processing Equipment:
Electropolishing is usually used on storage tanks, pipelines, valves, mixers, and food-contact surfaces. By eliminatingmicroscopic surface defects, electropolished parts can help to reduce bacterial and food residue adhesion, making the equipment easier to clean and sterilize.
Semiconductor and Electronics Industry:
Electropolishing is widely used to gas pipelines, valves, reactor linings, vacuum chamber components, and wafer handling equipment.
Aerospace and Nuclear Industry:
Since electropolishing can remove the mechanicalstress and enhance service life, it is usually applied to engine components, turbine blades, fuel nozzles, and internal reactor parts.
Consumer and Decorative Goods:
Electropolishing can help the surface achieve uniform and decorative appearance with durable gloss. It is commonly used to bathroom hardware, kitchenware, jewelry, and stainless-steel furniture fittings.
Automotive Parts:
Electropolishing is widely applied to automotive parts such as engine components, exhaust system parts, and decorative exterior trimsto reduce abrasion and enhance performance.
7. Best Practices for Electropolishing
- Plan for Heat Displacement
Heat displacement is one of the most issues during electropolishing process.
Since the application of electrical current would produce heat, the electropolishing tank must be sized properly for enough heat displacement of the electrolyte solution.
In addition, excessively small tank or big rectifier would hinder heat displacement. The heat might boil the solution or seriously damage the tank, and thus create a chemical burn hazard.
What’s more, the type of electrolyte can also influence heat displacement.
Most electrolytes are a solution mixed with phosphoric acid and sulfuric acid, which would hugely elevate temperature during the process.
The heat can lead to serious vapors that can respiratory issues and corrode the building interiors.
Therefore, adequate ventilation is also an important part for proper heat displacement. But if there is no condition for ventilation, glycol-based electrolyte is a good alternative chemistry.
- Correctly Design the Fixturing
The design of fixturing can significantly influence the distribution of electrical current.
It is important to correctly configure fixturing and racking to position the parts for optimal performance.
For instant, if you want to electropolish the internal surface of a pipe, you must make the pipe itself positive and run the negative cathode up inside the part.
But then dead short problem might occur due to the accident connecting with the positive and negative terminal.
Therefore, proper isolation points must be considered into the fixture design to prevent such connection.
More importantly, fixturing must be specifically designed based on individual part profile.
For complex components, it requires much more time and experimentation with prototypes to identify the best fixturing and blend of electrolyte chemistry.
- Mange the Hydrogen Bubbles
Excessive hydrogen bubbles along the surface would cause streaks on the finish. To solve this problem, you can reduce the current density to slow down the electropolishing process.
Or you can also try to adjust the fixturing of the parts. Using a proper mechanical method to pump the bubbles away from the surface is also a good solution.
- Remove Electrolyte Sludge Regularly
During the electropolishing process, electrolytes will generate certain sludge.
And the amount of generated sludge depends on the type of electrolyte. If the sludge tank is not be cleaned well, various problems and risks would happen.
For instant, if the generated sludge touches the cathodes, the electrical energy will be dissipated and then the finishing quality would be directly affected by it.
Additionally, the extra energy load can overload the rectifier, ultimately causing it to burn out. And if the heating unit is touched by the sludge, it might burn out.
Therefore, it is important to regularly remove the sludge out from the tank.
- Decant the Electrolyte Regularly
As the metal dissolves during the electropolishing, the concentration of metal ions of the electrolyte would be higher and higher.
These dissolved metal ions can alter the solution’s conductivity, viscosity, and chemical equilibrium.
What’s worse, they may even redeposit on the part surface and lead to defects such as haziness, dullness, and pitting, which would severely affect surface smoothness and brightness.
Additionally, some key active components would also be consumed during the process, which will reduce the polishing ability of the electrolyte.
As a result, electrolytes should be refreshed regularly.
However, it does not mean that you should replace all of the used electrolyte at any one time, except electrolytes with high amount of sludge.
You should only remove 10-20% of the solution and replace it with a fresh one to balance the concentration.
8. What are the Common Issues of Electropolishing and How to Resolve?
8.1. Appearing Pitting or Pinholes on the Surface
Causes
- Excessive concentration of impurities in the electrolyte
- Incomplete removal of surface contaminants such as oil or oxide scale
- Overly high current density or localized overheating
- Excessive electrolyte temperature or poor agitation
Solutions
- Regularly filter or replace the electrolyte to maintain low impurity levels
- Thoroughly clean the workpiece before polishing
- Optimize current density to avoid excessive values
- Maintain electrolyte temperature between 50–80°C and ensure sufficient agitation or circulation
8.2. Appearing Dull, Non-glossy, or Hazy
Causes
- Insufficient polishing time or current density
- Aged electrolyte or imbalanced composition
- Non-uniform material composition
- Failure to clean or passivate promptly after polishing
Solutions
- Extend polishing time or increase current density as needed
- Decant and replenish electrolyte components
- Use materials suitable for electropolishing
- Rinse immediately with deionized water after polishing and perform passivation treatment
8.3. Over Polishing at Edge or Corner
Causes
- Current concentrates at sharp edges or corners
- Improper electrode configuration, leading to excessively high local current density
Solutions
- Use auxiliary cathodes or shielding devices to balance current distribution
- Reduce overall current density and extend polishing time appropriately
- Perform rounding of sharp edges before electropolishing
8.4. Appearing Streaks or Flow Marks
Causes
- Poor electrolyte circulation causing gas bubbles to adhere to the surface
- Inadequate draining of electrolyte from the workpiece
- Insufficient agitation or improper fixture design
Solutions
- Enhance electrolyte circulation or agitation
- Optimize fixture angle to allow gas bubbles to escape smoothly
- Quickly transfer the workpiece to the rinsing bath after polishing to prevent drying marks
9. FAQs
9.1. What is the difference between electropolishing and passivation?
Electropolishing is an electrochemical process used to remove material from a metal surface, with the purpose of leveling, cleaning, and enhancing the metal’s inherent passivation properties.
Passivation is a chemical process primarily intended to remove free iron and surface contaminants, while forming a protective oxide film on the metal surface, without material removal.
For more information, visit: Electropolishing vs Passivation:Definition,Difference and How to Choose
9.2. How much material does electropolishing remove?
The material removal amount is controllable, depending on the desired level of surface smoothness and brightness. Typical removal is 5–50 microns (0.0005–0.003 inches).
9.3. Can electropolishing remove scratches or deep imperfections?
No. Electropolishing typically removes only 0.0005–0.003 inches (12–75 µm) of material. It smooths micro-roughness but cannot eliminate visible scratches, dents, or machining marks. For best results, parts should be pre-finished by grinding or buffing before electropolishing.
9.4. Does electropolishing improve corrosion resistance?
Yes—especially for stainless steel. By preferentially dissolving iron and other impurities from the surface, electropolishing enriches the chromium oxide layer, creating a more uniform, passive surface that resists rust and pitting. ASTM B912 and ASTM A967 recognize it as a valid passivation method.
9.5. Can electropolishing remove weld discolorationor heat tint?
Yes. Weld discoloration (heat tint) is a high-temperature oxide formed during welding. Electropolishing can effectively remove this tough oxide scale and discoloration, restoring the weld area to a smooth, clean, and corrosion-resistant state.
9.6. How does electropolishing differ from mechanical polishing?
Electropolishing is an electrochemical process that is used to dissolve and remove surface material at the microscopic level.
It achieves surface leveling and brightening without mechanical contact, making it ideal for complex geometries, internal surfaces, and hard-to-reach areas.
And there is no embedded abrasives or surface deformation, and mechanical stress with electropolishing.
Mechanical polishing is a mechanical process that smooths the surface through cutting or abrasion by grinding wheels, belts, or abrasives.
It levels the surface on a macroscopic scale, but may leave fine scratches, embedded abrasives, and residual stresses

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He’s a lifelong learner who loves sharing his expertise.
Other Articles You Might Enjoy

Electropolishing — Principles, Process, Advantages.
Electropolishing is an electrochemical process that removes microscopic surface peaks from metals, resulting in a mirror-like smooth finish, improved corrosion resistance, and reduced contamination risk.

A Practical Guide to Passivation for CNC Machined Parts 2025
Passivation chemically creates an ultra-thin, dense, and stable oxide film, transforming the metal from an “active” to a “passive” state. This process significantly enhances corrosion resistance without altering the part’s dimensions.

Electropolishing vs Passivation: Learn the Differences
Both electropolishing and passivation are common finishing techniques mainly used on stainless steels. They are both applied to improve the surface appearance and performance, but in different ways.

Chrome Plating VS Nickel Plating
Chrome plating and nickel plating are two common types of finishing techniques. They can both provide enhanced corrosion and abrasion resistance and an appealing appearance. The coatings plated on the part surface can make it more decorative and durable.


