Table of Contents
Nickel plating is widely used to enhance corrosion resistance, wear resistance, and appearance of metal and non-metal parts. This article provides a complete guide to nickel plating processes, types, and applications.
Therefore, this article will provide a detailed guide for nickel plating.
Key Takeaways:
Nickel plating coats a thin nickel layer via electroplating or chemical methods.
Two main types: Electroplating Nickel (EN) and Electroless Nickel Plating (ENP).
Choose EN for decorative or high-volume parts; ENP for wear-resistant, non-magnetic, or complex parts; black nickel for aesthetics or anti-glare.
1. What is Nickel Plating?
Nickel plating is a kind of finishing technology that coats a thin nickel film onto allowable parts through electroplating or chemical methods. It can effectively enhance the substrate’s corrosion resistance, wear resistance, and electrical conductivity, and also can impart an aesthetically pleasing metallic luster for the surface.
Nickel plating has a long history and has seen increasingly diverse applications over the decades. Its origins date back to Luigi Brugnatelli’s experiments in 1805.
Electroplating nickel was later developed by Golding Bird in 1837, and the most significant advancement came in 1916 with Oliver P. Watts’ invention of the Watts bath, combining nickel sulfate, nickel chloride, and boric acid into a reliable electroplating process.
Nickel plating has developed over the decades and still remains improved.
2. How Does Nickel Plating Work?
The core principle of corrosion protection by nickel plating lies in forming a continuous, dense, and chemically stable nickel coating on the substrate.
The coating would provide the plated part with both physical barrier and electrochemical protection.
The nickel’s chemical stability and structure optimization are also critical factors.
2.1. By Physical Barrier Protection
Physical barrier protection is the most fundamental corrosion protection provided by nickel plating.
The protection works by taking the nickel plating itself as a physical barrier to completely isolate the substrate from corrosive media such as oxygen, water, salt spray, acids, and alkalis.
The nickel plating forms a continuous and pore-free metallic film, or with low-porosity, on the substrate’s surface.
The film would serve as a protective coat to directly cut the contact between corrosive agents and the substrate, which can prevent oxidation or other chemical reactions at the source.
What is more, to enhance the isolation effect, process optimizations such as multi-layer nickel plating or pore-sealing treatments would be employed in practical applications to reduce coating porosity and improve the encapsulation for the substrate.
2.2. By Electrochemical Protection
The metal corrosion is essentially caused by electrochemical oxidation where the substrate gets corroded because of electron lost as the anode.
Nickel can provide electrochemical protection for its higher electrode potential compared to most common substrates such as iron and steel.
The main electrochemical protection is a cathode protection. If the coating is intact and pore-free, nickel itself would act as a cathode covering the substrate surface.
This prevents the substrate from contacting the corrosive medium to form an electrode pair, thereby halting the electrochemical corrosion reaction and directly inhibiting substrate corrosion.
The assistant electrochemical protection is a sacrificial anode protection. If the coating contains minor pores, the substrate would act as the anode, while the nickel coating would serve as the cathode.
At this moment, the nickel coating would preferentially lose electrons and be slowly corroded, thereby protecting the substrate from oxidation and providing indirect protection.
2.3. By Chemical Stability
Nickel is a chemically stable metal with excellent inherent corrosion resistance.
Firstly, nickel has good oxidation resistance at room temperature. Nickel does not readily react with oxygen in the air.
Instead, a thin and dense nickel oxide film would slowly form on its surface when touching with oxygen, which is non-porous and adherent.
The oxide layer can further enhance the chemical stability of the nickel coating and block reactions between the underlying nickel and external corrosive agents.
And nickel also has excellent resistance to acids, alkalis and salt spray. Nickel exhibits good tolerance to neutral salt spray and weakly acidic solutions.
It is only significantly corroded by strong oxidizing acids or highly alkaline environments, making it suitable for most industrial, marine, and daily applications.
2.4. By Structure Optimization
In practical nickel-plating processes, the corrosion protection performance is usually further improved by optimizing the coating structure.
Alloying modification and grain refinement are two common methods for structure optimization.
In alloying modification, elements such as phosphorus, chromium, or tungsten are commonly incorporated into the nickel coating to form nickel alloy coatings.
For example, electroless nickel-phosphorus alloy, with 8–12% phosphorus content, exhibits far superior corrosion resistance compared to pure nickel coatings.
Its amorphous structure can reduce corrosive penetration, and its phosphorus can enhance the coating’s chemical inertness, making it ideal for applications such as petrochemical and medical devices.
And the nickel plating’s grain structure can be refined by adjusting current density and brightener additives to make grains smaller and denser, which can reduce micro-porosity within the coating and further minimize the risk of corrosive infiltration.
3. Nickel Plating Types by Process
There are two kinds of key technical methods for nickel plating.
One is the electroplating nickel plating, also named as electrolytic nickel plating, and the other is the electroless nickel plating, a kind of chemical nickel plating.
3.1. Electroplating Nickel Plating
Electroplating nickel plating is fundamentally an electrolytic cell reaction.
By applying an external direct current (DC) power supply, nickel ions gain electrons and are reduced to metallic nickel at the cathode(the part to be plated), ultimately forming a uniform deposited coating.
3.1.1. Core Principle
This part will explain the system composition, electrode reactions, and plating formation of the electrolytic nickel plating.
- System Composition
A complete electrolytic system for nickel plating mainly consists of four core components.
Cathode: The part to be plated such as steel, copper, aluminum, or pre-treated non-metallic substrates like plastic. It is connected to the negative terminal of the power supply to serve as the site for nickel ion reduction and deposition.
Anode: Typically the high-purity nickel(purity≥99.9%). It is connected to the positive terminal of the power supply to dissolve and replenish nickel ions in the electrolyte, maintaining stable bath concentration.
Electrolyte: An aqueous solution containing nickel ions. Key components include nickel sulfate or nickel chloride to provide nickel ions, along with boric acid to stabilize pH and additives such as brighteners, leveling agents to improve coating appearance and performance.
DC Power Supply: To provide a stable current and control current density for plating thickness and uniformity.
- Electrode Reaction
Cathode Reaction(Reduction): Nickel ions (Ni²⁺) in the electrolyte gain electrons at the part’s surface and reduce to metallic nickel and then deposit on the surface(Ni²⁺ + 2e⁻ → Ni).
Anode Reaction(Oxidation): The metallic nickel anode dissolves and replenish nickel ions in the electrolyte(Ni → Ni²⁺ + 2e⁻).
- Plating Formation
As the electrolysis process continues, nickel atoms gradually accumulate on the surface, forming a well-adhered nickel coating with controllable thickness.
3.1.2. Plating Process
- Pre-process
Degreasing: Clean oil stains by alkaline solutions or organic solvents.
Pickling: Remove oxide scale with dilute hydrochloric or sulfuric acid.
Activation: Slightly etch the surface with dilute nitric or hydrochloric acid to create a micro-roughened surface for coating adhesion.
Rinsing: Rinse with purified water after each step to avoid contaminating the plating bath with residual chemicals.
- Electroplating
Place the pre-processed part into the plating tank as the cathode, opposite the nickel anode, and apply direct current. Key parameters are as follows.
Current Density: Typically 1-5 A/dm². Excessive current would cause roughness while insufficient current would slow deposition.
Temperature: 40–60°C for standard nickel plating and 60–80°C for high-phosphorus nickel plating.
pH Value: 3.5-5.0. It is usually stabilized by boric acid. And abnormal pH would cause blackening or peeling.
Time: Calculated based on target thickness.
- Post-process
Rinsing: Clean with purified water to remove residual electrolyte.
Drying: Use hot air or an oven(80–120°C) to eliminate moisture and prevent rust.
Passivation(Optional): Treat the coating with chromate solution to form a passive film for enhancement of corrosion resistance.
Polishing(Optional): Mechanically polish standard nickel coatings for gloss(not required for bright nickel plating).
- Quality Inspection
Visual Inspection: Check for pores, scratches, or blisters.
Thickness Measurement: Use magnetic thickness gauges for metallic substrates or eddy current gauges for non-metallic substrate to measure thickness.
Adhesion Test: Use cross-cut tape test or bend test to inspect adhesion.
Corrosion Resistance Test: Perform neutral salt spray test (NSS) to record the time until rust appears.
3.1.3. Advantages and Limitations
- Advantages
Excellent Protective Performance: The nickel coating can effectively isolate the substrate from air, water, and many other corrosive media to prevent rust.
Process Flexibility: By adjusting the electrolyte composition and current parameters, the thickness, gloss, and hardness can be precisely controlled.
Strong Compatibility: Electrolytic nickel plating can be applied to metals such as steel, copper, aluminum, and zinc, as well as metallized non-metallic surfaces.
- Limitations
Environmental Challenges: The electrolyte contains nickel ions(a heavy metal, classified as hazardous waste) and boric acid. It is required to strictly treat the wastewater to avoid environmental pollution.
Coating Defect Risk: Incomplete pre-treatment may lead to pinholes or peeling. And uneven current distribution can cause thinning on complex-shaped parts.
Limited Wear Resistance: The hardness of standard nickel coatings is lower than that of chromium plating. For long-term friction applications additional coatings are often required.
3.2. Electroless Nickel Plating
Electroless nickel plating, also know as chemical nickel plating, is a surface finishing technology that deposits a uniform nickel alloy coating on metal or non-metal substrates through chemical reduction reactions without relying on an external electric current.
3.2.1. Core Principle
The essence of electroless nickel plating is a redox reaction. The key lies on the autocatalytic characteristics. Below shows the table of core components for the reaction system.
| Component | Core Function | Common Substances |
|---|---|---|
| Metal Salt | To provide nickel ions (Ni²⁺) as the raw material for the coating | Nickel sulfate; Nickel chloride |
| Reducing Agent | To supply electrons for reducing Ni²⁺ to metallic nickel (Ni) | Sodium hypophosphite; Sodium borohydride |
| Complexing Agent | To combine with Ni²⁺ for forming stable complexes, preventing Ni²⁺ from hydrolyzing into nickel hydroxide precipitates and controlling the reaction rate | Citric acid; Lactic acid; Malic acid; EDTA |
| Buffer/Stabilizer | To maintain the stable pH of the plating bath and inhibit plating bath decomposition to avoid rough coatings caused by excessive reduction of Ni²⁺ | Acetic acid-sodium acetate buffer pair; Thiourea; Lead ions(trace amount) |
And the typical nickel plating solution formula is as follows(using sodium hypophosphite as the reducing agent).
Main Reaction(Nickel Deposition): Ni²⁺ + H₂PO₂⁻ + H₂O → Ni⁰↓ + HPO₃²⁻ + 3H⁺
Side Reaction(Phosphorus Deposition): H₂PO₂⁻ + H⁺ → P⁰↓ + H₂O + ½H₂↑
3.2.2. Plating Process
- Pre-process
Degreasing: Remove oil and grease from the substrate surface by solvent degreasing, alkaline chemical degreasing, or ultrasonic degreasing (suitable for complex parts).
Pickling/Activation: Remove surface oxide scale, rust, or oxide films to expose a fresh substrate surface and ensure coating adhesion.
Pre-process for Special Substrate:
For plastics like ABS and PC, it is required to sensitize the part first by immersing the part in stannous chloride solution to form an adsorption layer, and then activate the part by immersing it in palladium salt solution to generate a metallic palladium seed layer for catalytic sites.
For ceramics and glass, it is required to coat silane coupling agent first, followed by activation to enhance coating adhesion.
- Electroless Nickel Plating
Immerse the pre-processed part into a temperature-controlled bath (typically 85–95°C, and higher temperature for quicker deposition) while maintaining pH(4.5–5.5) and agitation to avoid local concentration gradients.
Calculate deposition time based on target coating thickness and replenish consumed nickel salts and reducing agents during plating to maintain bath stability.
- Post-process
Cleaning and Drying: After plating, rinse the part multiple times with deionized water to remove residual electrolyte and prevent corrosion. Then dry the part in an oven at 80–120°C to avoid water stains.
Heat Treatment(Optional): Anneal the dried part at 400–450°C in a vacuum or inert gas atmosphere for 1–2 hours to prevent oxidation. This can effectively enhance hardness and wear resistance.
Sealing Treatment(Optional): For extreme corrosion resistance demands, it is required to perform passivation or apply a clear coat to further enhance protection.
3.2.3. Advantages and Limitations
- Advantages
Excellent Coating Uniformity:
Electroless nickel plating is unaffected by uneven current distribution.
Even for deep holes, blind holes, grooves, and complex geometries, the thickness deviation can be controlled within ±5%(compared to >20% for electrolytic nickel plating), making it ideal for precision components.
Strong Adhesion:
The coating bonds with substrates via metallic bonding plus diffusion bonding, achieving adhesion strengths of 100–300 MPa, which far exceeds that of electroplating nickel plating(typically 50–150 MPa), and minimizing peeling risks.
Superior Corrosion Resistance:
Nickel-phosphorus alloy coatings exhibit an amorphous or microcrystalline structure with no grain boundary corrosion pathways.
In neutral salt spray tests, high-phosphorus coatings(8–12% P) can withstand 500+ hours without rust, making them suitable for marine environments and chemical equipment.
Controllable Hardness and Wear Resistance:
After heat treatment, phosphorus and nickel form Ni₃P hard phases, increasing hardness from Hv 500–600 to Hv 1000–1200(close to hard chromium coatings).
Compatibility for Non-conductive Substrate: Via pre-process, nickel can be plated on non-metallic surfaces.
- Limitations
Slower Deposition Rate:
Conventional acidic baths deposit at 10–20 μm/h(vs. 50–100 μm/h for electrolytic nickel) and thick coatings require prolonged plating, which would reduce production efficiency.
High Bath Cost with Short Lifespan:
Reducing agents usually consume rapidly and the accumulation of phosphite ions would inhibit reactions. Typically, only 5–10g of nickel per liter of bath can be deposited before disposal of bath, resulting in higher processing costs than electroplating nickel.
Internal Stress in Coatings:
High-phosphorus coatings, especially thick ones, may develop micro-internal stress. If the substrate lacks rigidity, this can lead to cracking.
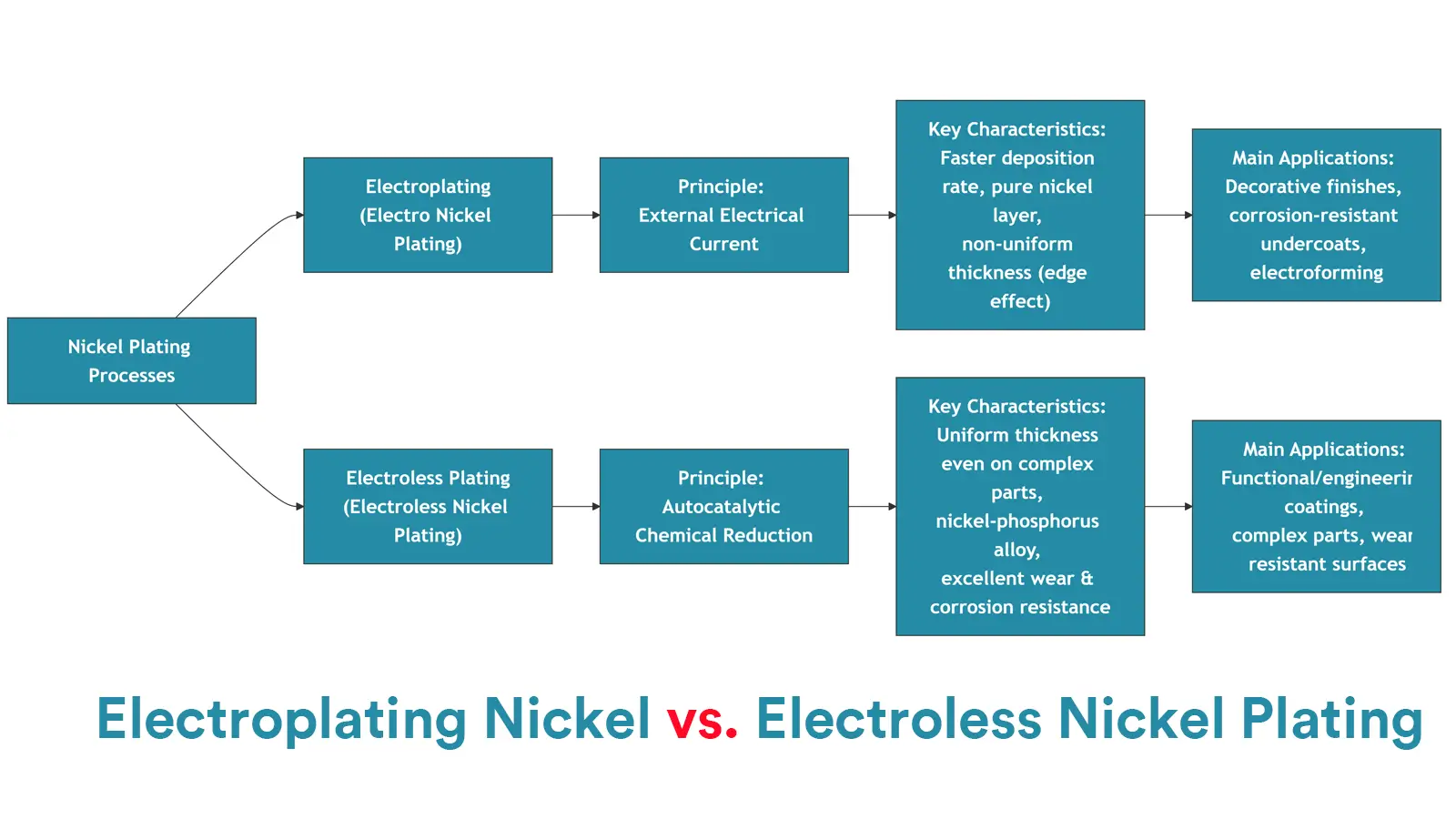
3.3. Electroplating Nickel Plating VS Electroless Nickel Plating
The main difference between electroplating nickel plating and electroless nickel plating lies on whether them should use external direct current supply or not. This difference would lead to many other detailed differences.
3.3.1. On Core Principles
The fundamental difference between the two processes lies on the driving force for nickel deposition, which directly determines their subsequent technological characteristics.
For electroplating nickel plating, the driving force depends on the external direct current power supply.
The core principle is to drive the reduction of nickel ions through electrode reactions, during which the part must be connected to the negative terminal of the power supply with electrical conductivity, serving as the cathode.
And then the electric field force would drive nickel ions to move toward the cathode and reduce them to metallic nickel.
However, electroless nickel plating does not require external current but depends on redox reactions with chemical reducing agents, during which the part only serves as the reaction substrate and then the reducing agent would provide electrons to directly reduce Ni²⁺ ions to Ni.
3.3.2. On Process Characteristics
Firstly, for electrolytic nickel plating, the part must be conductive.
Non-metal part must process a conductive layer first. While for electroless nickel plating, both metal parts and non-metal parts can be plated with nickel coating.
Secondly, the plating uniformity of electrolytic nickel plating is not as good as that of electroless nickel plating.
It is highly influenced by current distribution. For complex-shaped parts, problems of thick edges or corners with thin inner walls might appear to form poor uniformity.
While the plating uniformity of the electroless nickel plating is excellent since the nickel deposition only depends on chemical reactions but would not be constrained by shapes.
Thirdly, the nickel plating by electroplating commonly consists of pure nickel. But the electroless nickel plating is usually a nickel-phosphorus alloy coating.
3.3.3. On Key Features
For electroplating nickel plating, the hardness, corrosion resistance, wear resistance are all worse than that of electroless nickel plating since the electroless nickel plating contains phosphorus.
But all of these features can be improved through heat treatment.
However, the solderability of electrolytic nickel plating is better than that of electroless nickel plating.
Pure nickel coating exhibits excellent solderability, allowing direct soldering with high solder joint reliability.
While phosphorus within nickel-phosphorus alloys would tend to form brittle compounds during soldering.
Additionally, pure nickel is a kind of ferromagnetic material, and its coating exhibits good magnetism, making it suitable for applications requiring magnetic properties.
Low phosphorus electroless nickel plating also has magnetism but is poor.
While high phosphorus electroless nickel plating is completely non-magnetic, making it suitable for applications sensitive with magnetism.
Electroless vs. Electroplating Nickel Comparison Table
| Feature | Electroplating Nickel | Electroless Nickel Plating (ENP) |
|---|---|---|
| Process | Electrochemical | Autocatalytic Chemical |
| Power Source | External DC Power Required | No External Power Required |
| Deposit | Pure Nickel (Ni) | Nickel-Phosphorus Alloy (Ni-P) |
| Throwing Power | Poor (uneven coating on complex parts) | Excellent (uniform coating everywhere) |
| Primary Use | Decorative, Cost-Effective Protection | Functional, Wear/Corrosion Resistance |
| Hardness | Medium (160-400 HV) | As-Plated: High (500-700 HV); Heat Treated: Very High (~1000+ HV) |
| Magnetism | Magnetic | Non-Magnetic (with P content >8%) |
| Cost | Generally Lower | Generally Higher |
When to Choose Electroplating Nickel:
The application is for decorative finishes, cost-effective corrosion protection on simple shapes, and high-volume production.
When to Choose ENP?
Functional applications require uniform thickness on complex parts, superior wear resistance, enhanced corrosion resistance, and non-magnetic properties.
When to Choose Black Nickel?
Specialized decorative applications or functional anti-reflective surfaces where a dark matte finish is required.
4. Types of Electroplating Nickel
Depending on the composition of the plating solution, electroplated nickel can be categorized into bright nickel, dull nickel (Watts nickel), high-stress nickel, nickel sealing, and satin nickel.
4.1. Bright Nickel Plating
Bright nickel plating is produced through electrolytic nickel plating. By adding special chemicals, usually brighteners in solutions, the nickel film’s reflectiveness can be significantly enhanced and would be a polished, mirror-like finish.
Bright nickel plating stand outs especially for its decorative function. Therefore, it is popularly used in consumer products, automotive trims, lighting fixtures, and household items to make them visually appealing and meanwhile hard and corrosion-resistant.
However, it is required to carefully control bath composition and current density to prevent the nickel coating from pitting or uneven brightness.
4.2. Dull Nickel Plating(Watts Nickel)
When the surface is required to be non-reflective, dull nickel plating would come into play.
Without additional brighteners added in solutions, dull nickel plating would appear as a matte and low-gloss finish. It is usually applied to fuel systems, engine parts, and machinery components etc. where appearance is secondary to function.
Dull nickel plating can provide the part with a strong and adherent layer to enhance its mechanical properties.
More importantly, minor variations in bath conditions would not make so much effects on dull nickel plating as that on bright nickel plating.
4.3. Black Nickel Plating
Black nickel plating is with dark and matte finish. It is not pure nickel plating, but alloy or compound plating combined with zinc, copper, or other metals.
With unique black appearance, black nickel plating is commonly used in photography equipment, fashion accessories, and various consumer electronics.
One of the most critical advantages of black nickel plating is that its ornamental performance is much better than traditional chemical blackening methods and can provide moderate corrosion resistance meanwhile.
However, compared with other types of nickel plating, black nickel plating is not that much durable and wear-resistant.
The level would vary along with the exact bath composition and application process.
4.4. Nickel Sulfamate Plating
Nickel sulfamate plating is a kind of special electroplating nickel with high-purity and low-stress for primarily using nickel sulfamate as the main salt.
Through electrochemical methods, a dense, thick and uniform nickel coating would deposit on the substrate surface.
Its outstanding advantages include extremely low internal stress in the coating, excellent ductility, and high purity.
Therefore, it is widely applied in precision manufacturing fields such as aerospace part, electronic components, and mold processing, where good coating performance and excellent mechanical properties are required.
Additionally, with excellent adhesion and ductility, nickel sulfamate plating is also particularly suitable for parts requiring post-plating machining, during which chipping or flaking would not occur on the layer.
However, it is required to precisely control bath conditions, nickel concentration and pH levels for nickel sulfamate plating, making the process more complex and costly than basic electrolytic plating.
5. Types of Electroless Nickel Plating
Electroless Nickel Plating (ENP) can be classified into Low-Phosphorus ENP, Mid-Phosphorus ENP and High-Phosphorus ENP.
5.1. Low Phosphorus ENP
The mass fraction of phosphorus in low phosphorus electroless nickel plating is about 1% to 5%. Due to the low phosphorus content, the coating is exactly a crystalline coating, closely resembling pure nickel plating.
Its corrosion resistance is moderate, better than that of pure nickel plating and worse than that of high phosphorus electroless nickel plating.
And compared with high phosphorus electroless nickel plating, low phosphorus electroless nickel plating is suitable for applications requiring proper magnetic properties, solderability, electrical conductivity, and moderate corrosion resistance.
5.2. Mid-Phosphorus ENP
Mid-Phosphorus Electroless Nickel (EN) has a typical phosphorus content range of 6% to 9% by weight. It is the most widely used because of its excellent balance of corrosion resistance, hardness, and wear resistance. Non-magnetic.
Below is 1020 low carbon steel CNC parts with Mid-Phosphorus Electroless Nickel. 
5.3. High Phosphorus ENP
The mass fraction of phosphorus in high phosphorus electroless nickel plating is about 10% to 15%, making the coating be non-crystalline.
Therefore, high phosphorus electroless nickel plating is the hardest and most corrosion-resistant one in nickel plating. But it has poor solderability and electrical conductivity.
It is suitable for applications sensitive to magnetism and with strict requirement on corrosion resistance.
6. What are the Advantages of Nickel Plating?
•Good Corrosion Resistance: Nickel can form an extremely thin passive film of nickel oxide in the air. Being stable and dense, the film can block the substrate from corrosive media like water, oxygen, acids, and alkalis. Then the product’s service life is extended.
•Strong Wear Resistance and Hardness: Nickel plating makes the plated part much harder and more durable, helping the part withstand friction over time.
•Moderate Electrical and Thermal Conductivity: The electrical conductivity of nickel is about 22% of that of copper. And it also has stable thermal conductivity.
Therefore, nickel plating is widely used in electronic components such as connectors, PCB contacts, and battery tabs to prevent electrical performance from being impaired by oxidation and meanwhile to ensure stable transmission of current and heat.
•Proper Aesthetic Appeal: Nickel plating usually has smooth and stable finish. And its appearance can be controlled during the process.
•Excellent Adhesion and Ductility: Nickel exhibits strong adhesion to most metals, preventing delamination. And its ductility allows it to accommodate minor deformation without cracking.
7. What are the Applications of Nickel Plating?
•Industrial Machinery:
Industrial mechanical components are often exposed to friction, moisture, or chemical media. With good corrosion and wear resistance, nickel plating can effectively extend their service life.
Therefore, nickel plating is commonly applied to common mechanical components such as gears, bearings, bushings, valves, fasteners, and pump impellers and so on, as well as molding manufacturing for surface strengthening.
•Aerospace Parts:
With excellent corrosion resistance and hardness, nickel plating can hold up high temperature and stress, making it applied to engine parts and structural element in aerospace field.
•Electronics:
Electronic components require extremely high levels of electrical conductivity, soldering reliability, and electromagnetic compatibility.
Nickel plating particularly high-purity nickel perfectly meets these requirements, making it suitable for electronic connectors and terminals, PCB, and electromagnetic shielding components.
•Daily Items:
With polished and semi-bright appearance, nickel plating is commonly used in daily hardware and furniture such as faucets, door handles, cabinet pulls, lamp brackets, and metal furniture legs.
And it is also usually used for jewelry and handicrafts such as simulated silver jewelry, metal badges, commemorative coins, and toy accessories.
8. Nickel Plating on Different Materials
8.1. Nickel Plating on Steel
Steel is on of the most common materials for nickel plating. The steel itself is easy to be corroded and worn, and has poor hardness.
Nickel plating can provide a protectional film for steel, with excellent corrosion and wear resistance, as well as good solderability. Nickel plating steel is commonly used in automotive industry and electronic field.
8.2. Nickel Plating on Copper
Although an oxidation film would form on the copper when contacting with oxygen, it is still suitable for nickel plating since the oxidation film is brittle and can be cleaned by simple acid activation.
In addition, the adhesion between copper and nickel is excellent for their closely crystal structure. Nickel plating copper is popularly applied to electronic parts, PCB, and EMI shielding parts.
8.3. Nickel Plating on Aluminum
Nickel plating can also be processed on aluminum but not directly. The process is more difficult and complex than that of nickel plating steel and copper.
The reason is that there would form a thin dense and stable aluminum oxide on the aluminum surface when exposed to air, which would isolate aluminum and nickel. In addition, aluminum itself is much more active than nickel.
If plating is performed directly, a severe displacement reaction will occur and then a loose, porous, and poorly adherent nickel layer would deposit on the surface instead of a dense one.
Therefore, before doing nickel plating on aluminum, it is required to immerse the part in an alkaline zincate solution first, which can remove the aluminum oxide and form a dense zinc layer on the surface.
8.4. Nickel Plating on Stainless Steel
As we all know, the stainless steel itself is also with excellent corrosion resistance. Nickel coating would be plated on stainless steel part usually for specific appearance and solderability.
However, nickel can not be plated on stainless steel directly. It is required to remove the dense chromium oxide by strong acid activation first. And electroplating must be operated as long as the activation is done, after which a pre-plated nickel would form.
Nickel plating stainless steel is usually applied to precision manufacturing and medical devices.
8.5. Nickel Plating on Non-metals
The challenge of plating nickel on non-metals is that non-metals are non-conductive and inert. It is required to form a conductive metallic layer on the part through sensitization and activation first. Common non-metals for nickel plating include plastics, ceramics, and 3D printed parts.
9. How to Remove Nickel Plating?
•Acid Stripping Method:
This method is suitable for acid-resistant substrates such as steel and copper alloy parts. After cleaning the part with alcohol or acetone, immerse the part in the specific acid solution, during which the nickel plating would be removed gradually.
When the solution gradually comes into green and the part comes out, pick it put and clean it over time.
More importantly, note that acidic solutions are highly corrosive and reactions with nitric acid would produce toxic NO gas, the operation must be conducted in a fume hood.
•Alkaline Stripping Method:
This method is suitable for parts that are not acid-resistant such as aluminum, zinc alloys and magnesium alloys.
After warm the alkaline solution to 80-95°C, immerse the cleaned part in the solution and observe the reaction situation. The nickel plating would gradually turn black and stripped. When the original part comes out, pick it out and clean it.
•Grinding and Polishing Method:
This method is suitable for non-precision parts and those with thick nickel layers.
Firstly, heat the part with a blow dryer for adhesion reduction and grind off the majority of the nickel plating by coarse sandpaper.
Then polish the substrate with fine sandpaper or a polishing cloth wheel until the surface is smooth.
In summary, there are 3 ways to remove the Nickel plating: acid stripping, alkaline stripping and grinding.
10. Conclusion
With excellent corrosion resistance, strong hardness, proper conductivity, and good wear resistance, nickel plating plays a huge role in various industries such as automotive parts, daily hardware, and electronic parts etc.
If you are interested in nickel-plated parts, you can refer to relevant suppliers or consult professional manufacturers
11. FAQ
11.1. How Much Does Nickel Plating Cost?
The cost of nickel plating would vary along with the process, equipment, chemical solution and desired thickness. In addition, the material type matters too.
11.2. How Long Does Nickel Plating Typically Take?
The average nickel plating process would spend 30 minutes to several hours according to different requirements. The time particularly depends on the desired thickness, process method, and part dimension.
11.3. What is the Certificate for Nickel Plating?
ISO 4527 is the international standard for nickel plating.
11.4. How to Polish Nickel Plating?
Nickel plating can be polished by a soft cloth or a non-abrasive polish specifically for metals. Abrasive cleaners would damage the finish.
11.5. Can You Paint over Nickel Plating?
Yes, nickel plating allows for painting. But the surface should be lightly sanded and primed first to enhance the adhesion for painting.
11.6. Can You Power Coat over Nickel Plating?
Yes, nickel plating can also be power coated but it also requires stronger adhesion first.
11.7. Can You Solder to Nickel Plating?
Yes, nickel plating can be soldered. But due to the high melting point and high thermal conductivity of specific nickel plating, especially pure nickel plating, it is relatively challenge to solder to nickel plating. The soldering material and plating thickness do matter during the soldering.

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He’s a lifelong learner who loves sharing his expertise.

Zinc Plating - Everything You Shall Know
Zinc plating is a kind of surface finishing method for metal part protection, provides a barrier to prevent the surfaces from being directly exposed to moisture and oxygen.

Zinc Plating VS Nickel Plating
Both zinc plating and nickel plating are popular finishing method for metals. They can significantly improve corrosion resistance, abrasion performance, appearance, and certain functions of substrates.

What is Black Oxide Coating?
Black oxide, also known as Black Oxide or Blackening, is a surface finishing process that forms a dense black oxide coating on the surface of metals through a chemical reaction.

Phosphating Coating Guide
Phosphate conversion coating, also known as phosphating, is a surface treatment process that forms an insoluble phosphate protective film on the metal surface through a chemical conversion reaction.



