Table of Contents
Electroplating is a common surface finishing technique used in various industries for specific functions. It can provide the plated substrate with certain hardness, corrosion resistance, electrical conductivity, and wear resistance by plating a specific metal on its surface.
It is essential to have as much knowledge of electroplating as possible for the efficient production of metal parts.
Key Takeaways:
- This detailed guide explains what electroplating is, its core process (pre-treatment, plating, post-treatment), and the four common methods (rack, barrel, reel-to-reel, brush plating) with their pros, cons, and ideal applications.
- Explore the characteristics, purposes, and subtypes of six major electroplating finishes: Chrome (decorative vs. hard), Copper, Nickel (Watts, bright, black), Zinc, Gold (yellow, white, rose), and Silver.
- A clear breakdown of which materials (steel, aluminum, plastics, etc.) can be electroplated, the specific challenges for each, and the required pre-treatment processes. Includes practical notes on cost factors and surface preparation.
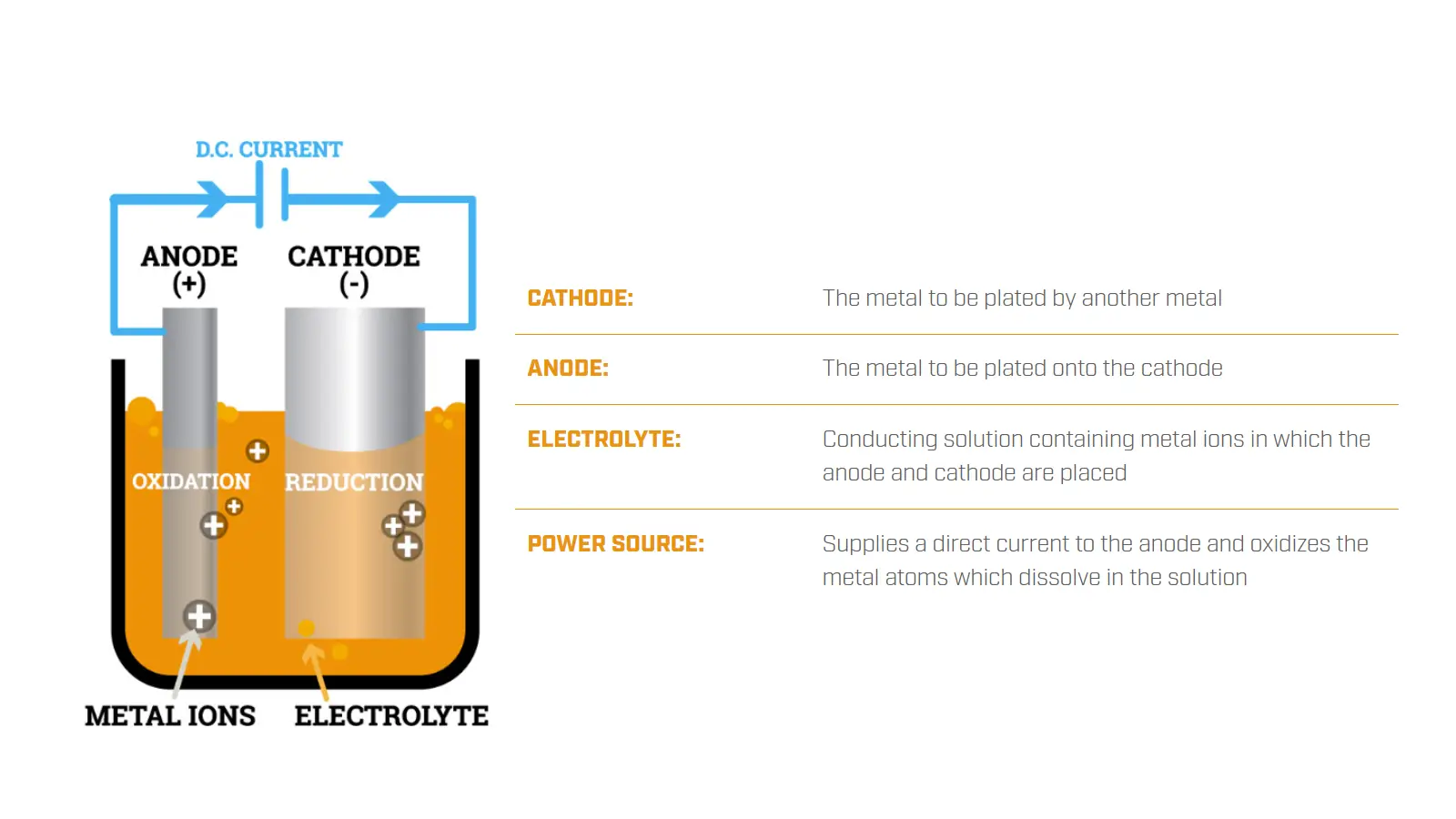
1. What is Electroplating?
Electroplating is a surface finishing process that involves coating one type of metal with a thin layer of another type of metal using an electric current. It is also known as electrodeposition.
Using the electrolysis principle, electroplating deposits a layer of metal or alloy on the surface to improve the material’s appearance, corrosion resistance, wear resistance, electrical conductivity, or other functional performance.
In fact, as long as the substrate is conductive, it can be electroplated.
Although metal remains the most common material for electroplating, as the autocatalytic coating technique advances, certain plastic parts can also be electroplated with metal layers.
During the electroplating process, the part to be plated serves as the cathode, while the plating metal serves as the anode. They will both be immersed in an electrolyte solution containing metal ions of the coating metal.
Actually, electroplating relies on redox reactions, during which one metal is oxidized at the anode while another metal is reduced at the cathode.
Once the direct current is applied, the metal cations in the solution will gain electrons at the cathode and be reduced to form a thin layer of metal coating.
At the same time, the metal atoms of the anode will be oxidized into ions and enter the solution as cations, replacing the deposited metal ions to maintain a stable concentration of metal ions in the electrolyte.
2. Types of Electroplating
Common electroplating types include chrome, copper, nickel, zinc, gold, and silver. Each one has its own features and specific applications.
2.1. What is Chrome Electroplating?
Since chromium is a chemically passive, dense, hard, and self-healing metal with a glossy luster, it is commonly used in metal electroplating to protect the metal substrate.
Chrome electroplating is usually divided into two types. One is decorative chrome electroplating, and the other is hard chrome electroplating. They are classified based on their different functions.
Decorative chrome electroplating is primarily used to make the part bright and mirror-like, enhancing its appeal. The coating is extremely thin on the top.
There are typically a copper undercoat and a nickel mediate coat beneath the chrome coat.
In fact, it is the nickel plating that provides the primary corrosion resistance, while decorative chrome plating is primarily used to further enhance the surface’s ability to prevent oxidation and discoloration.
Decorative chrome electroplating is commonly applied to automotive parts, such as wheels and grilles, as well as to sanitary ware, furniture, and consumer products.
Compared to decorative chrome coating, hard chrome electroplating is primarily used to enhance the surface’s mechanical properties, including hardness, corrosion resistance, abrasion resistance, and friction coefficient.

Hard chrome coating is typically much thicker than decorative chrome coating and is applied directly to the part surface.
It is commonly used on industrial machinery components, such as cylinder pistons and rollers, molds, tools, and other similar applications.
The plating solutions for chrome electroplating are typically divided into hexavalent chromium solutions and trivalent chromium solutions.
With severe toxicity, the former one is strictly restricted in many countries and areas. While the latter one is more environmentally friendly.
Related blog: What is Chrome Plating: Definition, Types, Process, Cost, Process and Application
2.2. What is Copper Electroplating?
Copper electroplating is also one of the most common electroplating methods. There are many advantages of copper electrocoating.

Firstly, copper electroplating can effectively enhance the part’s electrical conductivity.
Copper is an excellent electrical conductor and is widely used in electronic components, connectors, and printed circuit boards (PCBs) to enhance electrical conductivity.
Secondly, copper coating typically serves as an undercoat for other metal coatings within a multi-layer system. It can provide excellent adhesion and a smooth plate for subsequent coatings.
Thirdly, copper electrocoating can improve the surface’s ability to transfer heat.
And the solution systems for copper electroplating have three common types.
The most common one is sulfate copper plating, whose solution is composed of copper sulfate, sulfuric acid, chloride ions, and additives such as brighteners and levelers.
This system is commonly applied to PCB through-hole plating and as an undercoat layer for fast deposition, excellent conductivity, and low cost.
However, with certainly poor dispersibility, its coverage on complex parts is usually uneven, requiring additives to improve distribution and uniformity.
The second one is cyanide copper plating, whose solution is composed of copper cyanide, sodium cyanide, and sodium hydroxide.
Due to its good adhesion, this system is usually used as an undercoat for active metals such as steel and zinc alloys.
However, the solution is highly toxic. It has been gradually phased out by strict environmental regulations.
The final one is pyrophosphate copper plating, whose solution is composed of copper pyrophosphate and potassium pyrophosphate. This system is cyanide-free and environmentally friendly.
Furthermore, it exhibits good dispersibility, allowing for uniform coating on complex parts. However, the solution is certainly not stable. It needs a high cost for strict control.
2.3. What is Nickel Electroplating?
There are three common types of nickel electroplating.
The primary one is Watts nickel plating, whose solution is typically composed of nickel sulfate, nickel chloride, and boric acid. The coating is usually bright or semi-bright, providing an appealing decorative effect.
Furthermore, due to its good ductility and adhesion, nickel electroplating often serves as an intermediate or base layer for subsequent coatings.
The second one is bright nickel electroplating.

With the addition of organic brighteners to the standard plating solution for Watts nickel, a brightly mirror-like surface can be obtained directly without the need for polishing. Therefore, it is widely used for decorative components in sanitary ware and household appliances.
The third one is black nickel electroplating whose solution is typically composed with nickel salt, zinc salt, sulfur compound, and boric acid. The coating is typically dark gray to black based on the solution formulation and current density.
And the coat has low reflectivity, so it is commonly used to optical instruments, military, and aerospace.
Moreover, its distinctive matte or satin black appearance can provide elegant and subtle decoration for electronic products and decorative items.
Related blog: What is Nickel Plating?
2.4. What is Zinc Electroplating?
The plating solution for zinc electrocoating is usually zinc sulfate, zinc chloride, or cyanide zinc.
Compared with other metal coatings, zinc coating is a kind of sacrificial protection. Even if the coating is locally damaged, the exposed steel can still be protected by the remaining zinc. This can improve the part’s durability and service life.
In addition, zinc plating can provide various appealing appearance. The coating surface is usually smooth and can be designed in patterns and passivated in different colors.
What’s more, the zinc electrocoating can bond tightly to the base metal. Even during operations such as rolling, drawing, or bending, that can alter shapes, the coating is not prone to peeling or damage.
And zinc is also a conductive metal coating.
Color zinc plating is primarily distinguished by the surface color formed after passivation, including blue-white zinc, yellow (rainbow) zinc, and black zinc.

 These colors do not represent different zinc-plating processes, but rather the passivation treatments applied to the zinc layer after electroplating.
These colors do not represent different zinc-plating processes, but rather the passivation treatments applied to the zinc layer after electroplating.
Zinc electroplating is widely applied to the automotive industry, electronics, and construction hardware.
Related blog: Zinc Plating – Everything You Shall Know
2.5. What is Gold Electroplating?
There are three common types of gold electroplating based on the appearance and composition.
The primary one is yellow gold electroplating. Gold is the most classic and common color for gold electroplating.
Yellow gold electroplating typically uses high-purity gold and sometimes adds small amounts of silver or copper to finely adjust color and hardness.
It typically exhibits a warm, bright yellow tone reminiscent of natural gold. And due to its chemical stability, pure gold is extremely resistant to oxidation and discoloration.
Therefore, pure gold electroplating is widely used in jewelry and accessories, such as necklaces, rings, and watches.
What’s more, pure gold has extremely low electrical resistance.
As one of the best conductive materials, pure gold is commonly used in wire bonding, electronic connectors, and semiconductor applications.
And pure gold is much softer, with poor wear resistance.
Note: Based on our experience with pure gold plating, CNC parts require careful polishing before the plating process. Otherwise, the surface will appear relatively rough after gold plating. Additionally, the price of gold plating is closely linked to the gold market price, and the thickness of the gold plating layer also affects the final cost
The second one is white gold electroplating. It is designed to mimic the appearance of natural platinum. Platinum electroplating is expensive, so it is rarely used for ordinary decorative purposes.
Actually, white gold electroplating is a type of alloy coating whose gold plating solution typically contains white metals, such as nickel and palladium.
It typically appears silver-white or bright white with a cool-toned luster, offering a more modern look compared to traditional yellow gold.
Additionally, to achieve a brighter and purer white appearance, rhodium plating is usually applied to many white gold coatings. Rhodium is extremely hard and highly resistant to oxidation.
Among white gold electroplating, the Au–Ni alloy is the most common type, which contains 75%–90% gold with the remainder being nickel , providing a white color and hardness.
While Au–Pd alloy is the most high-end option, offering better corrosion resistance, it is commonly used in elevated jewelry to avoid nickel allergies.
Among the three types of gold electroplating, white gold electroplating often exhibits the highest wear resistance, thanks to the excellent scratch resistance provided by rhodium plating.
The hardness and corrosion resistance of white gold electroplating are also excellent. It exhibits worse electrical conductivity than pure gold, but remains acceptable.
The third one is rose gold electroplating. It has become increasingly popular in recent years, favored for its soft and romantic hue. Rose gold electroplating is a type of alloy composed of gold and copper. It typically exhibits a soft pink or reddish gold hue, falling between the tones of yellow gold and copper.
The color of rose gold electroplating is achieved by adjusting the copper content in the gold plating solution. Higher copper proportion leads to a deeper red hue.
The standard rose gold plating, also 18k gold electroplating, is typically composed of 75% Au, 22.25% Cu, and 2.75% Ag. It is the most common rose white electroplating, widely used for watches, wedding rings, and mobile phone accessories.
While the 22k rose gold electroplating is pink gold electroplating with softer and more elegant appearance. It is usually composed with 91.7% gold, 7-8% copper, and 0.5-1% Ag, commonly applied to luxury jewelry and accessories.
And the 14k rose gold electroplating typically appears deep red or copper red with high saturation. It is often composed of 58.3% gold, 35-40% copper, and 1-2% Ag.
With a higher copper content, the coating’s hardness would be enhanced, and its color would be more intense; however, it is also prone to oxidation and darkening. It is also widely used to fashion accessories at a much lower cost.
The hardness and abrasion resistance of rose gold electroplating are worse than those of white gold electroplating and better than those of yellow gold electroplating.
While its corrosion resistance is worse than that of both white gold and yellow gold. Copper is easier to oxidize and also has higher electrical resistance.
2.6. What is Silver Electroplating?
During the common silver electroplating process, the part to be plated acts as the cathode, and a piece of pure silver acts as the anode.
They are both immersed in an electrolyte containing silver ions, typically a potassium silver cyanide solution. When the direct current is applied, the silver ions are reduced and deposited on the surface.
There are various features of silver electroplating. Firstly, silver has the highest electrical conductivity among all metals.
Therefore, silver plating is widely used in electrical and electronic components to enhance the conductivity of contacts and connectors.
Additionally, silver exhibits has good solderability, which facilitates connections for electronic components.
Secondly, with a specially appealing luster and good corrosion resistance, silver electroplating is widely used for tableware, jewelry, handicrafts, and musical instruments.
Thirdly, silver has high reflectivity for visible and infrared light, so silver electroplating is widely used in reflecting mirrors and optical devices.
However, if the silver is exposed to an environment containing sulfur, black Ag₂S forms on the surface.
Silver is soft with normal wear resistance, usually coated with nickel to improve performance.
Types of Electroplating: Core Characteristics Table
| Plating Type | Definition / Primary Purpose | Key Characteristics & Mechanism | Common Classifications / Types |
|---|---|---|---|
| Chrome Plating | Deposits chromium to provide corrosion resistance, hardness, and decorative finish. | Chemically passive, very hard, dense, glossy. Offers excellent wear resistance and a self-healing oxide layer. | 1. Decorative Chrome: Thin top layer for appearance; requires Cu/Ni undercoats. 2. Hard Chrome: Thick, direct deposit for engineering properties like hardness and wear resistance. |
| Copper Plating | Deposits copper for excellent electrical/thermal conductivity or as an adhesive undercoat. | Superior electrical and thermal conductor. Provides a smooth, level base for subsequent plating layers. | By Bath Chemistry: 1. Sulfate Acid Copper: Fast, economical. 2. Cyanide Copper: Excellent adhesion. 3. Pyrophosphate Copper: Cyanide-free, good throwing power. |
| Nickel Plating | Deposits nickel for corrosion protection, durability, and as a versatile barrier layer. | Good corrosion/wear resistance, ductility, and hardness. Can be plated to various finishes. | 1. Watts Nickel (Semi-Bright): Standard for corrosion protection. 2. Bright Nickel: Contains brighteners for a mirror finish. 3. Black Nickel: Dark coating for low reflectivity. |
| Zinc Plating | Deposits zinc primarily for sacrificial (galvanic) corrosion protection of steel. | Acts as a sacrificial anode, corroding preferentially to protect the underlying steel substrate. | By Passivation Color (post-treatment): – Blue-White Zinc – Yellow (Iridescent) Zinc – Black Zinc |
| Gold Plating | Deposits gold for superior corrosion resistance, highest conductivity, and prestigious decoration. | Extremely corrosion-resistant, highly conductive, chemically inert. Soft in pure form. | By Alloy/Appearance: 1. Yellow Gold: Classic, high purity. 2. White Gold: Au alloyed with Ni, Pd, etc. 3. Rose Gold: Au alloyed with Cu for pink/red hue. |
| Silver Plating | Deposits silver for the highest electrical conductivity, solderability, and lustrous finish. | Best electrical/thermal conductivity of all metals. Good solderability and reflectivity. Tarnishes in sulfur environments. | Typically classified by application (e.g., electrical, decorative, optical). |
3. What is the Process of Electroplating?
3.1. Pre-process
- Degreasing: To remove organic contaminants such as grease, fingerprints, and polishing compoundsby alkaline cleaners.
- Rinsing: Rinse the part with deionized water to thoroughlyremove any remaining degreasing agent.
- Pickling: Immerse the part in a diluted acid solutionto remove scale, rust, and light corrosion. Note to control the immersion time to avoid over-etching.
- Rinsing: Completely rinse the part with pure water to remove any residual acid to prevent contamination of subsequent process.
- Activation: Briefly immerse the part in a mild acid solutionto remove any passive film on the surface. It can ensure the metal is in an active state to improve the adhesion of the subsequent plating or coating.
3.2. Electroplating
- Racking: Mount the part onto a conductive rack, ensuring proper electrical contact and uniform current distribution.
- Electroplating: Connect the part to be plated and the plating metal with a direct current supply, and then place them into the plating bath containing specific electrolyte. The part serves as the cathode while the plating metal serves as the anode.
Composed with the similar metal intended for deposition, the anode acts as the sacrificial source of metal ions. Through controlled anodic dissolution, it can provide positively charged metal ions to the surrounding electrolyte.
And because of the cathode’s negative charge, it would attract the positively charged metal ions within the electrolyte bath. Then a thin layer of metal coating would deposit on the surface.
The electrolyte must be conductive to ensure the proper flow of electricity between the anode and cathode. In addition, it usually contains a dissolved salt of the metal to be deposited for continuous supply of object metal ions.
What’s more, it is crucial to strictly control the key processing parameters such as current density, temperature, pH level, and agitation, which would significantly influence the plating rate and quality.
3.3. Post-process
- Rinsing: Rinse the part multiply to remove any residual plating solution for no contamination or corrosion.
- Passivation (Optional): Passivate the part with specific solution for higher corrosion resistance.
- Drying: Dry the part completelyby hot air to prevent residual
- Sealing/Coating (Optional): Apply a clear coating to the partby spraying, or immerse the part in an anti-tarnish solution.
- Inspection: Check the appearance and certain performance of the coating.
4. What are the Common Methods for Electroplating?
There are four types of common methods for electroplating. Each of them has special features, advantages, and disadvantages. It is suggested to choose the proper one based on the products.
4.1. Rack Plating
By rack plating, a single part or multiple parts will be secured on the conductive rack. Then the whole rack will be suspended in the plating bath as the cathode.
As the current is applied, electroplating develops. In addition, the rack is usually specifically designed.
Features of Rack Plating
- Each part is positioned individually, allowing precise control of current distribution.
- The parts must contact with the rack well for good electrical connection, but it might leave marks on the surface.
- Rack plating is suitable for multilayer electroplating processes.
Advantages of Rack Plating
- Rack plating can often produce high-quality coating with uniform thickness and good appearance.
- Rack plating is suitable for complex and large precision part.
- The process is easy to monitor and automate.
Disadvantages of Rack Plating
- Rack plating needs manual racking, leading to low efficiency and high labor cost.
- The contact points might leave marks that require post-plating.
- Rack maintenance cost relatively high.
- Applications: Automotive trim and door handles; bathroom hardware such as faucets and shower heads; aerospace structural components; high-end electronic device housings
4.2. Barrel Plating
For barrel plating, a large quantity of small parts would be placed into a perforated rotating barrel ((typically hexagonal or cylindrical in shape and made of PP, PVC, or titanium).
Then the barrel will be immersed in the plating bath as the cathode. As the barrel slowly rotates, the parts tumble continuously for uniform metal deposition.
Features of Barrel Plating
- The process can achieve self-agitation since the parts would collide and tumble against each other.
- Electrical current is delivered to the parts through the perforated conductive barrel wall or internal cathode contact rods.
- Barrel plating systems include horizontal barrels and vertical barrels. Vertical barrels are more suitable for parts that tend to tangle or interlock.
Advantages of Barrel Plating
- The production efficiency is certainly high for large-volume manufacturing.
- The overall cost is low, withno need for expensive racks or complex loading/unloading procedures.
- The tumbling motion provides a mild polishing effect on the parts due to continuous rolling and friction, without rack marks on the surface.
Disadvantages of Barrel Plating
- The coating uniformity is inferior to rack plating, with thinner coating on internal holes, deeps grooves, and recessed areas due to shielding effect.
- Barrel plating is only suitable for small parts with simple shape. It is note suitable for components that are fragile or easily deformed, which would be damaged due to mutual collision among the parts.
- Barrel plating is also not suitable for precision parts or components that tend to tangle.
- Applications: Fasteners such as screws, nuts, and washers; small electronic terminals and spring contacts; toy components and watch parts; small hardware pieces such as zipper sliders and buckles etc.
4.3. Reel-to-Reel Plating
Reel-to-reel plating is a highly automated plating method specifically designed for continuous strip or wire materials. During the process, coiled metal strips or wires would be continuously fed into the plating bath vis guide rollers for coating.
Features of Reel-to-Reel Plating
- The processingline speeds can achieve from a few meters per minute to several tens of meters per minute.
- Coating thickness is precisely controlled by the current density and line speed.
- Parallel anode plates or jet plating are commonly used to improve coating uniformity.
Advantages of Reel-to-Reel Plating
- Reel-to-reel plating is highly automated with high efficiency and consistency, suitable for large-scale production.
- The coating quality is stable with precise control on thickness.
- The process can achieve selective plating on specific areas for material saving.
Disadvantages of Reel-to-Reel Plating
- Reel-to-reel plating is only applicable for regularstrip or wire materials.
- The initial investment is high for complex equipment.
- The process is not much flexible. It needs to adjust production line for different products.
- Applications: Connector terminal strips; battery tabs; lead frames etc.
4.4. Brushing Plating
Brushing plating is a specialized portable electroplating method that does not require immersing the entire part in the electrolyte.
For brushing plating, a plating brush dipped with plating solution would be moved locally over the part surface that serves as the cathode.
As the direct current is applied to the part, it will be selectively electroplated in the plating solution. The plating brush is typically made of graphite, stainless steel, or cotton-wrapped anode.
Features of Brushing Plating
- The process does not require full immersion, allowing on-site operation.
- The plating solution is typically with high-concentrationand high-conductivity.
- The plating area and thickness is able to be controlled.
Advantages of Brushing Plating
- The process is flexible and portable, ideal for localized maintenance and reinforcement.
- The process does not affect other areas of the part.
- Brushing plating is suitable for extra-large and non-removable parts.
Disadvantages of Brushing Plating
- The low efficiency is not suitable for high-volume production.
- The processing effectiveness is highly dependent on the operator’s skills.
- Its plating smoothness may be inferior to tank plating.
Applications:
Repairment for shaft parts such as hydraulic rodsand motor shafts; Local requirement for molds; on-site maintenance for large equipment such as ships and wind turbine gears; surface hardening for printing rollers etc.
5. Which Material can be Electroplated and Not?
The key to electroplating lies in the material’s electrical conductivity.
Most metal materials is conductive and can be electroplated. Some passive metals should be activated first before electroplating to enhance adhesion.
What’s more, for certain materials without electrical conductivity, chemical plating should be applied first to make them conductive.
- Steel and Iron:
Steel materials such as carbon steel, stainless steeland cast iron are the most commonly electroplated materials, typically coated with zinc, nickel, or chromium to improve corrosion resistance and decorative appearance.
- Copper and Copper Alloys:
Copper materials such as brass and bronze have excellent electrical conductivity. They are commonly used as an undercoat or directly plated with gold or silver for electronic components.
- Aluminum and Aluminum Alloys:
Aluminum is a kind of passive metal on which there would always be a hard and passive oxide layer. Such a passivated coating would lead to poor adhesion for direct electroplating.
Therefore, special pre-processes such as zincate treatment or anodizing plating are required before electroplating.
- Zinc andZinc Alloys:
Zinc and its alloys are commonly used for decorative electroplating on die-cast parts in automotive and hardware industries.
They typically require a multiple coating system, a copper layer first, and then a nickel layer, finally followed by a decorative or functional chrome coating.
- Magnesium and Magnesium Alloys:
Magnesium and magnesium alloys are important in the aerospace and automotive industries due to their low weight.
They are electroplatable but require highly sensitive pretreatment and intermediate layers due to their active surface.
There would always be an oxidation layer(MgO) on the magnesium surface when exposed to water or air, which is insulating and has poor adhesion.
In addition, magnesium is much easily to be dissolved or corroded by plating solutions, especially acidic ones.
Before electroplating, it is required to pretreat magnesium parts primarily to change its active surface and plate a strike coating on the surface.
Firstly, the oxide film on the magnesium surface must be removed through mild pickling by special chromic acid or phosphoric acid solutions. And note to avoid excessive attack in the meantime.
Secondly, immerse the part in a weakly alkaline or neutral zinc salt solution. A thin and dense zinc immersion layer would form on the magnesium surface through a chemical displacement reaction.
The two steps can help to isolate the magnesium surface from the plating solutions and prevent corrosion, and to provide a conductive and well-adhering intermediate layer.
Thirdly, electroplate a non-acidic underlayer, typically cuprous pyrophosphate or mildly alkaline nickel on top to seal and protect the zinc layer since the zincate layer is very thin and still highly reactive.
Note to plate a properly thick coat to ensure complete isolation between the magnesium substrate and the main plating solutions.
Finally, the common electroplating processes can be applied to the part.
- Titanium and Titanium Alloys:
Titanium and its alloys are widely used in aerospace and medical implants due to their excellent hardnessand corrosion resistance, as well as light weight.
Electroplating is applied to titanium parts usually for special functions such as good electrical conductivity and friction
However, it is certainly difficult to electroplate titanium parts. Titanium would instantly form a very dense and chemically stable titanium dioxide film(TiO₂) in air.
This layer is highly insulating and chemically inert, which fundamentally prevents strong adhesion for electroplating.
The key to electroplate titanium lies on removing the oxide layer and establish an instantaneous conductive path.
It is required to use highly corrosive agents for pickling to dissolve or thin the oxide layer. And the process must be strictly controlled to prevent over-etching.
Then a copper or nickel undercoat is needed. You can take advantage of the strong complexing ability of fluoride ions to deposit a copper or nickel plating on the surface at extremely elevated temperature.
Or you can used special nickel or copper strike plating by short current to deposit metal atoms rapidly on the surface at the instant when the oxide layer is dissolved.
Finally, you can apply common electroplating on the surface.
- Plastics:
Plastics are becoming increasingly popular in the industry for their lightweight nature and low cost, although metals remain the dominant materials.
However, nowadays, a combination of 3D-printed parts with electroplating is increasingly used in various industries for design freedom and good protection.
Certain plastics, such as ABS, PC/ABS, and PP plastics, are electroplatable as long as they are properly pretreated.
Below is a photo of plastic 3D-printed parts in decorative chrome electroplating.
The key to electroplating plastics lies in electrical conductivity.
There are three crucial steps before electroplating 3d prints.
Firstly, it is necessary to chemically etch the plastic part by immersing it in a strong oxidizing solution, typically a mixture of chromic acid or sulfuric acid.
This would create tiny and uniform pits on the surface to enhance the mechanical adhesion between the part and plated layer.
Secondly, activate the part by immersing it in a solution containing palladium and chloride ions.
The palladium ions would be adsorbed onto the microporous surface and reduced to colloidal palladium nuclei. The surface is then uniformly covered with catalytically active palladium particles.
Thirdly, electroless plating must be applied to the plastic surface to make it conductive. It is required to immerse the part in a plating solution containing nickel or copper salts.
The metal ions in the solution would be reduced to metal atoms under the catalytic action of the palladium nuclei and deposit on the surface as an entire layer.
Therefore, the plastic surface is now coated with a thin, uniform layer of copper or nickel, transforming the surface from an insulator to a conductor.
Finally, common electroplating can be applied to the plastic surface. However, there are also some plastics that cannot be electroplated, such as PE, PTFE, PVC, PS, and PC plastics.
| Material | Can It Be Electroplated? | Why / Key Properties | Required Pretreatment | Notes |
|---|---|---|---|---|
| Steel & Iron (Carbon Steel, Stainless Steel, Cast Iron) | Yes | Conductive; most commonly electroplated materials | Standard cleaning | Typically coated with zinc, nickel, or chromium to improve corrosion resistance and appearance |
| Copper Materials (Brass, Bronze) | Yes | Excellent electrical conductivity | Usually none needed | Commonly used as an undercoat or plated directly with gold or silver |
| Aluminum & Aluminum Alloys | Yes (requires pretreatment) | Passive oxide layer causes poor adhesion | Zincate treatment or anodizing plating | Special processes required before electroplating |
| Zinc & Zinc Alloys | Yes | Commonly used for decorative electroplating | Copper layer → Nickel layer → Chrome layer | Used for die-cast parts in automotive and hardware industries |
| Magnesium & Magnesium Alloys | Yes (very difficult) | Forms MgO layer which is insulating and easily corroded | Mild pickling → Zinc immersion layer → Non-acidic copper/nickel undercoat | Requires thick underlayers to prevent corrosion during plating |
| Titanium & Titanium Alloys | Yes (extremely difficult) | Instantly forms dense TiO₂ insulating film | Strong pickling → Copper or nickel strike plating | Requires establishing conductive path instantly after oxide removal |
| Plastics (ABS, PC/ABS, PP) | Yes (after processes) | Non-conductive; must be activated first | Chemical etching → Palladium activation → Electroless copper/nickel | Common for decorative chrome on 3D-printed parts |
| Plastics (PE, PTFE, PVC, PS, PC) | No | Poor adhesion and not suitable for electroless coating | — | These plastics cannot be electroplated |
6. What are the Common Pros and Cons of Electroplating?
6.1. What are the Advantages of Electroplating?
- Enhancing Corrosion Resistance:
Electroplating coatings can effectively isolate the base metal from the corrosive media such as water, oxygen, and salts to lengthen the part’s service life.
- Improving Appearance:
Electroplating can make the part’s surface much brighter, smoother, and more uniform. Gold electroplating, silver electroplating, bright nickel and decorative chrome electroplating are usually used to make the part more appealing.
- Enhancing Hardness and Wear Resistance:
Certain hard coatings such as hard chrome plating, nickel phosphorus alloys, or composite electroplated coatings, can significantly increase the surface hardness and wear resistance of parts, making them suitable for components that must withstand friction.
- Improving Electrical Conductivity:
Plating metals that are highly conductive, such as gold, silver or copper, onto electronic components or connectors can reduce electrical resistance and ensure the reliability and efficiency of signal transmission.
- Restoring Dimension:
By depositing additional metal on the part’s surface, electroplating can help to increase the dimensionof worn part.
6.2. What are the Disadvantages of Electroplating?
- Environmental Impact:
It is known that strong acids and alkalis as well as heavy metal salts are commonly used in electroplating processes.
If the wastewater and sludge are not properly treated, they can cause severe environmental pollution.
This requires companies to invest in costly equipment and treatment for environmental protection.
- Poorer Uniformity:
For parts with complex geometries, the currentdistribution would usually be uneven, which can lead to thicker coating at sharp edges while thinner or even absent coating at recesses.
- Hydrogen Embrittlement:
During the electroplating process, hydrogen atoms may diffuse into the substrate, leading to hydrogen embrittlement, especiallyfor high-strength steels.
This would cause worse resilience and higher brittleness. Therefore, specific treatment to remove hydrogen is required to mitigate this issue for no cracking risk.
- Higher Cost:
Electroplating processes often involve long processing time, complex bath formulations, strict temperature and current control, and the use of expensive raw materials. As a result, the overall cost tends to be relatively high.
7. Summary
In summary, electroplating is a versatile surface finishing technology essential across modern manufacturing. This guide has covered the core electroplating process, practical methods (rack, barrel, reel-to-reel, brush), and key plating types like chrome, nickel, zinc, gold, and silver—each serving distinct functional or decorative purposes.
A crucial insight is that electroplating extends beyond traditional metals. With specialized pretreatment, materials like aluminum, titanium, and certain plastics (e.g., ABS) can also be plated, enabling innovative applications such as plated 3D-printed parts.
While offering significant benefits in corrosion resistance, hardness, conductivity, and aesthetics, electroplating also involves considerations like cost, environmental impact, and technical challenges such as coating uniformity.
Successful implementation depends on selecting the appropriate plating type and method based on your material, part design, and performance needs. Armed with this knowledge, you can make informed decisions to effectively utilize this powerful technology.
8. FAQs
8.1. What is Reverse Electroplating?
Reverse electroplating is actually electropolishing during which a thin metal layer would be dissolved from the surface by direct current. While electroplating is a deposition process.
8.2. What is Platinum Electroplating?
Platinum electroplating is a electrochemical process during which a thin layer of platinum metal would deposit on the base surface.
Platinum is a rare precious metal that has extremely excellent corrosion resistance to nearly every chemical substance. It also has brilliant conductivity, catalytic activity, and biocompatibility. What’s more, it appears bright white with appealing appearance.

Lucas is a technical writer at ECOREPRAP. He has eight years of CNC programming and operating experience, including five-axis programming. He’s a lifelong learner who loves sharing his expertise.
Other Articles You Might Enjoy

What is Chrome Plating?
Chrome plating, or chromium electroplating, is a surface finishing technology that deposits a layer of chromium onto a metal or plastic surface through an electrolytic process.

Zinc Plating - Everything You Shall Know
Zinc Plating are classifed into Hot-Dip Galvanizing, Electro Zinc Plating,Mechanical Zinc Plating and Electroless Zinc Plating.

What is Nickel Plating?
Nickel plating is a kind of finishing technology that coats a thin nickel film onto allowable parts through electroplating or chemical methods. Two main types: Electroplating Nickel (EN) and Electroless Nickel Plating (ENP).

What is Electropolishing?
Electropolishing is an electrochemical process that removes microscopic surface peaks from metals, resulting in a mirror-like smooth finish, enhanced corrosion resistance, and reduced risk of contamination.


